A case of primary aldosteronism with rhabdomyolysis in which the first symptoms were thyrotoxicosis and peripheral paralysis and literature review
Highlight box
Key findings
• Provide clinical ideas for correct diagnosis and decision-making of primary aldosteronism (PA).
What is known and what is new?
• PA is one of the main causes of especially refractory hypertension.
• We report and review the characteristics of some atypical and uncommon special case cases related to proaldosterone.
What is the implication, and what should change now?
• We report a seemingly simple case, which actually has many special characteristics.
• A young man with hypokalemia and thyrotoxicosis is easily misdiagnosed as Graves’ disease in clinic; after careful investigation, we found that PA is the real cause of hypokalemia. Thyrotoxicosis is caused by thyroiditis rather than hyperthyroidism; in addition, whether thyroiditis or hypokalemia, it is rare to cause rhabdomyolysis in clinic. We sorted out the complicated clinical data of this case and systematically reviewed the relevant literature, which provided reference for the correct clinical strategy of similar cases.
Introduction
Primary aldosteronism (PA) is one of the main causes of secondary endocrine hypertension, especially refractory hypertension (1,2). Increased voluntary secretion of aldosterone leads to increased blood pressure and proinflammatory effects on different organ systems, including the cardiovascular system (3,4). At present, there is no detailed literature clarifying the association between PA and thyroid function. Rhabdomyolysis (RM) is a syndrome characterized by muscle necrosis and the release of muscle cell contents into the blood circulation. There have been individual reports of RM caused by severe hypokalemia in PA patients in the past; however, whether the occurrence of RM is affected by the regulation of thyroid hormones is unclear. Therefore, we report such a case, and based on it, we conducted a review of the relevant literature to further explore the clinical features of thyroid dysfunction combined with PA and RM to guide the diagnosis and treatment of the disease. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-103/rc).
Case presentation
A 38-year-old male patient was admitted to the emergency department of the First Affiliated Hospital of Baotou Medical College on June 19, 2020, due to lower limb fatigue and soreness for 5 days that was aggravated for 2 days. Two days before coming to the clinic, the symptoms of lower limb fatigue and soreness worsened, and the patient found it difficult to get up in bed. The patient was admitted to Ordos City Hospital, where a blood potassium of 2.27 mmoI/L (reference value: 3.5–5.5 mmol/L) was recorded. Thyroid stimulating hormone (TSH) was 0.01 mIU/mL (reference value: 0.27–4.2 mIU/mL), free tetraiodothyronine (FT4) was 34.2 pmol/L (reference value: 12–22 pmol/L), and serum potassium after potassium supplementation was 2.75 mmol/L (reference value: 3.5–5.5 mmol/L). The symptoms did not improve; therefore, the patient presented to the emergency department of our hospital, where his blood potassium was measured at 2.59 mmol/L (reference value: 3.5–5.5 mmol/L). According to the patient’s self-reported medical history, there was no special discomfort in the past, and he had not had regular health checkups, and was not found to have elevated blood pressure, nor had he taken any medication. From the emergency department, he was admitted to the endocrinology department with hypokalemia.
Physical examination on admission: temperature 36.2 ℃, pulse 98 beats/min, respiration 20 breaths/min, blood pressure 146/96 mmHg. The thyroid was not noticeably swollen, the patient did not report tenderness, and there were no abnormalities in the heart, lungs, or abdomen. No swelling of the lower limbs was noted. Neurological: the examination of the four limbs muscle strength is weakened, upper limb muscle strength grade 3, bilateral lower limb muscle strength grade 1, muscle tone is not abnormal, bilateral biceps and triceps tendon reflexes are normal, bilateral knee and Achilles tendon reflexes are weakened. Cardiac ultrasound showed that the left atrium of the heart was large and the left ventricular wall was thickened with small amount of regurgitation of the aortic valve and tricuspid valve, and the rest of the cardiac ultrasound did not show any obvious abnormalities.
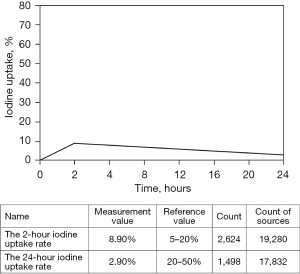
After admission, relevant laboratory tests were performed (Table 1). The results show TSH was decreased, triiodothyronine (T3) and tetraiodothyronine (T4) were increased, and thyroid peroxidase (TPO), thyroglobulin (Tg), and thyrotropin receptor antibody (TRAb) were negative. Thyrotoxicosis was present and was not caused by Graves’ disease. Ultrasound of the thyroid revealed calcification in the right lobe and a hypoechoic area in the left lobe. Further examination revealed that the iodine uptake rate was 8.9% at 2 hours (h) and 2.9% at 24 h (Figure 1), indicating reduced iodine uptake, which was consistent with the changes in iodine uptake observed in subacute thyroiditis.
Table 1
| Project title (reference value) | Time | |||||||
|---|---|---|---|---|---|---|---|---|
| 2020-06-18 | 2020-06-19 | 2020-06-20 | 2020-06-21 | 2020-06-22 | 2020-06-23 | 2020-06-24 | 2020-06-28 | |
| K (3.5–5.5 mmol/L) | 2.25 | 2.59 | 2.29 | 2.7 | 3.24 | 3.67 | 3.93 | 3.92 |
| Na (135–155 mmol/L) | 148.1 | 145.0 | 141.0 | – | – | – | – | – |
| Cl (96–106 mmol/L) | 98.8 | 103.9 | 89.8 | – | – | – | – | – |
| Ca (2.15–2.50 mmol/L) | – | – | 1.95 | 2.14 | – | – | – | – |
| P (0.85–1.51 mmol/L) | – | – | 0.79 | 0.77 | – | – | – | – |
| Mg (0.75–1.02 mmol/L) | – | – | 0.72 | 0.76 | – | – | – | – |
| TSH (0.27–4.2 mlu/mL) | 0.01 | 0.01 | – | – | – | – | – | – |
| FT4 (12–22 pmol/L) | 34.2 | – | – | – | – | – | – | – |
| T4 (58.1–144.6 nmol/L) | – | 201.80 | – | – | – | – | – | – |
| T3 (0.92–2.79 nmol/L) | – | 2.24 | – | – | – | – | – | – |
| TPO-Ab (0–34 IU/mL) | – | 34 | – | – | – | – | – | – |
| Tg-Ab (0–115 IU/mL) | – | <15 | – | – | – | – | – | – |
| TRAb (0–2.0 IU/mL) | – | 0.30 | – | – | – | – | – | – |
| RBC (4.3×1012/L–5.8×1012/L) | – | 5.01 | – | – | – | – | – | – |
| WBC (4×109/L–10×109/L) | – | 11.47 | – | – | – | – | – | – |
| Platelet (100×109/L–300×109/L) | 424 | 424 | – | – | – | – | – | – |
| The ratio of neutrophils (50–75%) | 76.11 | – | – | – | – | – | – | – |
| ANC (1.8×109/L–6.3×109/L) | – | 8.55 | – | – | – | – | – | – |
| LYM (20–40%) | 17.82 | – | – | – | – | – | – | – |
| LYMPH (20–40%) | 17.9 | – | – | – | – | – | – | – |
| EOSR (0.5–5%) | 0.30 | – | – | – | – | – | – | – |
| EO (0.5–5%) | – | 0.2 | – | – | – | – | – | – |
| GLU (3.9–6.1 mmol/L) | – | – | 5.1 | – | – | – | – | – |
| BUN (3.1–8.0 mmol/L) | – | – | 2.3 | – | – | – | – | – |
| Cr (57–97 μmol/L) | – | – | 78 | – | – | – | – | – |
| Uric acid (208–428 μmol/L) | – | – | 267 | – | – | – | – | – |
| CO2 (20–29 mmol/L) | – | 27.7 | – | – | – | – | – | |
| Blood cortisol (4.26–24.85 ng/dL) | – | – | – | – | 9.16 (8:00) | 13.33 (8:00) | – | – |
| Blood cortisol (2.5–15.8 ng/dL) | – | – | – | – | – | 2.93 (0:00) | 1.41 (0:00) | – |
| ACTH (7.2–63.3 ng/L) | – | – | – | – | 19.33 (8:00) | 10.23 (0:00) | – | – |
| ACTH (7.2–63.3 ng/L) | – | – | – | – | – | 26.97 (8:00) | 5.30 (0:00) | – |
| Epinephrine (plasma) (42.36–163.16 pg/mL) | – | – | – | – | – | – | – | 35.63 |
| Epinephrine (urine) (0–80 nmol/24 h) | – | – | – | – | – | – | – | 5.59 |
| Norepinephrine (plasma) (195.2–357.8 pg/mL) | – | – | – | – | – | – | – | 160.86 |
| Norepinephrine (urine) (0–590 nmol/24 h) | – | – | – | – | – | – | – | 51.71 |
| Dopamine (plasma) <20 pg/mL | – | – | – | – | – | – | – | 55.64 |
| Dopamine (urine) (107.2–246.6 μg/day) | – | – | – | – | – | – | – | 319.23 |
K, potassium; Na, sodium; Cl, chlorine; Ca, calcium; P, phosphorus; Mg, magnesium; TSH, thyroid stimulating hormone; FT4, free tetraiodothyronine; T4, tetraiodothyronine; T3, triiodothyronine; TPO-Ab, thyroid peroxidase antibody; Tg-Ab, anti-thyroglobulin antibody; TRAb, thyrotropin receptor antibody; RBC, red blood cell; WBC, white blood cell; ANC, absolute neutrophil count; LYM, lymphocyte ratio; LYMPH, lymphocyte percentage; EOSR, eosinophil ratio; EO, eosinophil; GLU, glucose; BUN, blood urea nitrogen; Cr, creatinine; CO2, carbon dioxide; ACTH, adrenocorticotropic hormone.
The patient had muscle soreness, weakness, tawny urine discharge, weakened muscle strength of the limbs during physical examination, grade 3 muscle strength of the upper limbs, grade 1 muscle strength of both lower limbs, normal muscle tone, normal reflexes of brachial and triceps tendons bilaterally, weakened reflexes of the knee and Achilles tendons bilaterally, and significantly increased myoglobin and muscle enzyme. According to the medical history, symptoms, signs, and laboratory examination, RM was diagnosed. Considering that hypokalemia may be the main cause, the symptoms were significantly improved after active potassium supplementation, alkali supplementation, hydration, and other comprehensive treatment, and the laboratory indicators gradually normalized (Table 2). Monitoring 24-h ambulatory blood pressure revealed that the average 24-h daytime and nighttime blood pressure values were elevated, the maximum daytime and nighttime blood pressure values were elevated, and the circadian rhythm disappeared. Cardiac ultrasound: left atrial diameter (LAD) =40 mm, interventricular septal thickness at diastole (IVSD) =13 mm, interventricular septum thickness at end-systole (IVSS) =7 mm, left ventricular posterior wall in diastole (LVPWD) =12 mm, left ventricular posterior wall thickness at end-systole (LVPWS) =9 mm, left ventricular end diastolic dimension (LVDD) =48 mm, left ventricular systolic diameter (LVDA) =32 mm, ejection fraction (EF) =61%, fractional shortening (FS) =32%, Epeak =0.6 m/s, Apeak =0.7 m/s, main pulmonary artery (MPA) =20 mm, pulmonary artery flow rate =0.9 m/s, ascending aorta =30 mm, aortic flow rate =1.0 m/s. Tissue Doppler imaging: E'v/A'v <1, Doppler ultrasound measurement of tricuspid flow velocity [continuous wave of the tricuspid regurgitation (CW TR)]: Vmax =2.0 m/s, pressure gradient (PG) =16 mmHg, pulmonary artery systolic pressure (PASP) =21 mmHg. Doppler echocardiography revealed increased mitral valve flow during the diastolic period and a small amount of regurgitation across the aortic and tricuspid valves. Although the patient had not been previously found to have elevated blood pressure, after the patient is admitted to the hospital, ophthalmic fundus examination indicated arteriovenous tortuosity in both eyes, artery thinning, and the presence of binocular arteriosclerosis. The abnormal parameters indicated target organ manifestations of hypertension. The patient has a history of hypertension without knowing it and has had it for some time. Blood gas analysis: blood pH =7.52 (reference value: 7.35–7.45), partial pressure of carbon dioxide (PCO2) =39 mmHg (reference value: 35–45 mmHg), partial pressure of oxygen (PO2) =67 mmHg (reference value: 95–100 mmHg), K+ =2.5 mmol/L, HCO3− =31.8 mmol/L, base excess (BE) =8.3 mmol/L, SO2 =95%, synchronous urine pH =7.5, negative urine glucose, negative urine ketone bodies, and negative urine protein. A serum potassium of 2.98 mmol/L was measured simultaneously with the 24-h urine potassium of 110.60 mmol/24 h, suggesting the possibility of potassium loss via the renal route. Therefore, screening for the etiology of endocrine-related hypokalemia combined with hypertension was needed. The measurement of serum cortisol and adrenocorticotropic hormone (ACTH) rhythm showed that serum cortisol was 2.93 µg/dL at 0:00 during sleep, and urine free cortisol was 50.02 nmol/24 h at 24 h. However, when the patient was hypokalemic and was receiving continuous fluid rehydration for RM, the interference of stress and other factors could not be excluded. Re-examination after the normalization of blood potassium revealed a normal of serum cortisol and ACTH rhythm; the serum cortisol at 0:00 during sleep was 1.41 µg/dL, and the 24-h urine free cortisol was 37.8 nmol/24 h. Therefore, there was insufficient evidence of hypercortisolism. After the weakness and soreness of the lower limbs had significantly improved, the blood and urine catecholamine levels normalized and pheochromocytoma and paraganglioma were ruled out.
Table 2
| Time | Myohemoglobin (μg/L) | Troponin (ng/mL) | AST | LDH (U/L) | CPK (U/L) | CK (U/L) | CK-MB (U/L) | HBDH (U/L) | K (mmol/L) | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory range | <110 | <0.04 | 0–40 | 100–300 | 18.0–198 | 18–198 | 0–18 | 90–182 | 3.5–5.5 | – |
| 2020-06-19 | 1,032.5 | 0.03 | – | – | 8,189 | – | 70.1 | – | 2.75 | (4 hours) Rehydration was 1,500 mL, potassium chloride 9 g, sodium bicarbonate 5 g |
| 2020-06-20 | 707.3 | 0.01 | 92 | 335 | 6,868 | 8,189 | 70.1 | 232 | 2.29 | 9,000 mL of fluid, potassium chloride 21 g, sodium bicarbonate 10 g |
| 2020-06-21 | 301.8 | – | 76 | 245 | – | 4,751 | 46.3 | 176 | 2.7 | Fluid replacement was 6,000 mL, potassium chloride 15 g, sodium bicarbonate 10 g |
| 2020-06-22 | 135.8 | – | – | – | – | – | – | – | 3.24 | Fluid replacement was 6,000 mL, potassium chloride 12 g, sodium bicarbonate 10 g |
| 2020-06-23 | – | – | – | – | 1,827 | – | 36.3 | – | 3.67 | Rehydration was 3,000 mL, potassium chloride 16 g, sodium bicarbonate 10 g |
| 2020-06-24 | 91. 1 | – | – | – | – | – | – | – | 3.93 | Fluid 2,000 mL, potassium chloride 3 g, sodium bicarbonate 10 g |
| 2020-06-25 | – | – | – | – | – | – | – | – | 3.85 | Fluid 2000 mL, potassium chloride 3 g, sodium bicarbonate 10 g |
| 2020-06-26 | 41 | – | – | – | – | – | – | – | 3.38 | Rehydration was 2,250 mL, potassium chloride 3 g, sodium bicarbonate 10 g, spironolactone 20 mg ×3/day orally |
AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CPK, creatine-phosphokinase; CK, creatine kinase; CK-MB, creatine kinase isoenzyme; HBDH, α-hydroxybutyrate dehydrogenase; K, potassium.
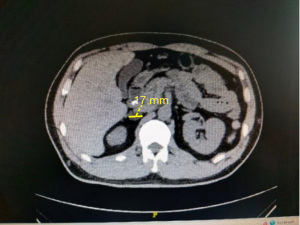
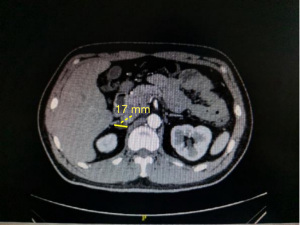
After the correction of hypokalemia, renin-angiotensin-aldosterone system (RAAS) hormone levels were measured on June 23: renin =2.32 pg/mL (reference value: 4–38 pg/mL), angiotensin II =167.77 pg/mL (reference value: 49–252 pg/mL), aldosterone =178.90 pg/mL (reference value: 40–310 pg/mL), cortisol =9.16 ng/dL (reference value: 4.26–24.85 ng/dL), ACTH =19.33 ng/L (reference value: 7.2–63.3 ng/L), and aldosterone-to-renin ratio (ARR) =7.71 when the direct renin concentration (DRC; ng/L) was measured according to the 2008 American Endocrine Society Guidelines for the treatment of PA published in JCEM journal (5). The cut-off ARRs in different centers were 3.8, 5.7, and 7.7, and the screening of this patient was positive. The repeated ARR was 8.43 in the screening test, and the result remained positive. Meanwhile, repeat the measurement of the RAAS system and calculate the ARR ratio results remained positive. One-hour aldosterone was 196.22 pg/mL, the inhibition rate was 20%, and 2 h aldosterone 211.93 pg/mL, the inhibition rate was 13.6%. The inhibition rate of serum aldosterone after the captopril test was lower than 30% of the cut-off point that was recommended by the United States guidelines. At the same time, 2 h after the administration of aldosterone 21.193 ng/dL (Table 3), which also exceeded the cut-off of more than 11 ng/dL recommended by domestic experts (6), supporting the diagnosis of primary hyper aldosteronism. Furthermore, the aldosterone level decreased after orthostasis, and possibility of an aldosteronoma was considered in the differential diagnosis. Computed tomography (CT) scan of the patient’s adrenal gland revealed a round, low-density shadow with a clear edge and a size of about 17 mm × 11 mm in the right adrenal gland, indicating a right adrenal adenoma (Figure 2). On enhanced scan, a round, low-density shadow with a clear edge and a size of about 17 mm × 11 mm was observed on the right adrenal gland; it was mildly and uniformly enhanced on the enhanced scan, indicating a right adrenal adenoma (Figure 3).
Table 3
| Time | K (mmol/L) | Renin (pg/mL) | AngII (pg/mL) | Ald (pg/mL) | COR (ng/dL) | ACTH (ng/L) | ARR |
|---|---|---|---|---|---|---|---|
| 06.23; upright position | 3.67 [3.5–5.5] | 2.32 [4–38] | 167.77 [49–252] | 178.90 [40–310] | 9.16 [4.26–24.85] | 19.33 [7.2–63.3] | 7.71 |
| 06.24; upright position | 3.93 [3.5–5.5] | 1.88 [4–38] | 149.19 [49–252] | 158.35 [40–310] | 7.21 [4.26–24.85] | 18.54 [7.2–63.3] | 8.40 |
| 06.24; supine position | – | 1.74 [4–24] | 127.76 [25–129] | 163.49 [10–160] | 13.33 [4.26–24.85] | 26.97 [7.2–63.3] | 9.40 |
| 06.25; before taking medicine | – | 2.55 [4–38] | 176.46 [25–129] | 245.24 [10–160] | – | – | – |
| 06.25; 1 hour after administration | – | 2.33 [4–38] | 182.46 [25–129] | 196.22 [10–160] | – | – | – |
| 06.25; 2 hours after administration | – | 2.62 [4–38] | 177.42 [25–129] | 211.93 [10–160] | – | – | – |
Ranges in the parentheses are reference values. K, potassium; AngII, angiotensin II; Ald, aldosterone; COR, cortisol; ACTH, adrenocorticotropic hormone; ARR, aldosterone renin ratio.
In summary, the patient was diagnosed as PA adrenocortical adenoma (aldosteronism adenoma), PA secondary hypertension, PA secondary hypokalemia, RM, and subacute thyroiditis. After multidisciplinary team (MDT) consultation with indications and considerations for surgery, laparoscopic resection of the right adrenal tumor was performed on June 30, 2020. During the operation, the adrenal gland was freed, the peripheral vessels were clamped using a titanium clamp and a Hem-o-lok clamp, and the adrenal gland was exposed. An adrenal adenoma was found with a size of about 17 mm, and the adenoma was completely removed after the complete free adenoma was gradually completed. The adrenal gland was clamped using a Hem-o-lok clamp, and no bleeding was detected in the adrenal area. The specimen was placed in a specimen bag and removed through a small incision in the skin. The gauze instruments were checked, a drainage tube was placed, and the incision was closed by layer.
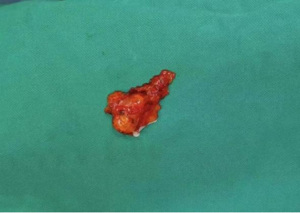
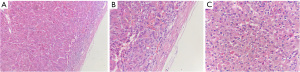
The resected specimen was a grayish-yellow non-plastic tissue 3 cm × 2 cm × 0.8 cm in size. The surface was partially light and partially rough, the section was nodular, apricot yellow, and the boundary was clear (Figure 4). Histopathology indicated an adrenal cortical adenoma (Figure 5).
Postoperative spironolactone 20 mg was given orally once a day, and the patient’s blood pressure was monitored. One month after the surgery, the patient was followed up in the outpatient department, Electromyography showed normal nerve conduction, motor and sensory nerve conduction in the upper and lower extremities, normal F wave amplitude, and normal repetitive nerve stimulation (RNS) wave amplitude and a blood pressure of 125/85 mmHg was recorded. The three levels of thyroid activity were in the normal range, the myoenzyme was normal, the blood potassium was 4.35 mmol/L, and the oral administration of spironolactone was stopped. Three months after surgery, blood pressure was measured at 118–126/79–88 mmHg and blood potassium was 3.98 mmol/L. Over the following 2 years, the blood pressure did not deviate significantly from the previously reported values, and no specific discomfort was reported by the patient. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The prevalence of primary hyperaldosteronism is high, accounting for approximately 17–23% of refractory hypertension and an ultra-high 4.0% incidence of newly diagnosed hypertension. The danger of PA is high, and studies have found that excess aldosterone is an important risk factor for cardiac hypertrophy, heart failure, and impaired renal function. Compared to patients with essential hypertension, damage to hypertensive target organs, such as the heart and kidneys, is more severe in patients with PA.
PA involves the spontaneous secretion of aldosterone by the adrenal cortex, resulting in sodium retention and potassium excretion, increased blood volume, and inhibition of RAAS activity. PA can induce excessive potassium excretion, In Asian patients with hypertension, the prevalence of PA is 5–15% (7); the prevalence is higher in treatable hypertension. Only a small proportion of patients (9–37%) develop hypokalemia, mostly in severe cases, leading to RM under extreme conditions (6).
In this case, the main manifestation was muscle weakness on admission, and the electrolyte test showed severe hypokalemia, and the kinase spectrum was significantly increased, so the patient was diagnosed with RM. At the same time, the comprehensive examination confirmed the existence of hypertension and caused corresponding changes in the fundus arteries, indicating that the course of hypertension in this patient is not short, that is, the course of PA is not short, and the tolerance of most PA patients to hypokalemia increases with the prolongation of the course of disease. However, this patient suddenly developed muscle soreness, weakness, and RM before the visit. We suspected that the patient’s thyroid hormone metabolism disorder increases the magnitude of hypokalemia and accelerates the development of hypokalemia.
“Primary aldosteronism” and “Rhabdomyolysis” were used as the keywords to search all relevant literatures in CNKI database, VIP database, Wanfang database and PubMed. Seventy-three articles and 31 individual cases met the requirements (summarized in Table 4). In RM caused by hypokalemia, the hypokalemia is the result of rapid and mass transfer of potassium from the extracellular space to the intracellular space, mostly into muscle. This is believed to be related to the increased activity of the sodium/potassium adenosine triphosphatase (Na/K ATPase) pump, which influences the synthesis and storage of cellular glycogen, resulting in insufficient cell energy supply, metabolic disorders of muscle cells, deterioration of cell membrane stability and increased permeability, and the release of creatine kinase (CK) and other harmful substances into the blood, resulting in RM.
Table 4
| Number | Gender | Age (years) | Blood pressure on admission (mmHg) | CK (U/L) | K (mmol/L) | Renin | Ald | Pathology |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 36 | 146/96 | 8,189 | 2.59 | 2.55 pg/mL | 245.24 pg/mL | Adenoma |
| 2, (8) | M | 41 | 144/99 | 11,359 | 1.8 | <0.5 μIU/mL | 198.0 pg/mL | Adenoma |
| 3, (8) | F | 51 | 142/89 | 10,897 | 1.8 | <0.5 μIU/mL | 2,230 pg/mL | Adenoma |
| 4, (9) | F | 65 | 120/87 | 18,370 | 1.8 | – | >1,000 pg/mL | Adenoma |
| 5, (10) | M | 53 | 164/110 | 15,930 | 2.1 | 1.3 pg/mL | 38.8 ng/dL | Adenoma |
| 6, (10) | M | 46 | 217/136 | 1,629 | 1.9 | – | 13.6 ng/dL | Adenoma |
| 7, (11) | F | 55 | 144/90 | 2,900 | 1.5 | 4 µU/mL | 209 pg/mL | Adenoma |
| 8, (11) | F | 21 | 105/65 | 1,454 | 1.9 | – | – | N |
| 9, (12) | F | 33 | 189/93 | – | 1.9 | 0.5 ng/mL/h | 0.5 ng/mL/h | N |
| 10, (13) | F | 42 | 150/75 | 1,579 | 2.1 | – | 424.6 pmol/L | Adenoma |
| 11, (14) | F | 48 | 160/90 | 14,248 | 1.3 | 0.27 ng/mL | 887 pg/mL | Adenoma |
| 12, (15) | F | 30 | 120/80 | 45,720 | 1.6 | – | – | N |
| 13, (16) | M | 45 | 180/84 | 20,451.94 | 1.4 | – | – | Adenoma |
| 14, (17) | F | 18 | – | 16,838 | – | – | – | Adenoma |
| 15, (18) | M | 40 | 163/110 | 5,788 | 3.1 | <0.1 μg/L/h | 124.10 ng/L | Adenoma |
| 16, (19) | F | 51 | 184/96 | 6,168 | 1.37 | 0.02 ng/mL/h | 271.5 pg/mL | N |
| 17, (20) | F | 51 | 175/104 | 29,700 | 2.56 | 138.2 pmol/L/h | 272.9 pmol/L | Adenoma |
| 18, (21) | F | 27 | 189/134 | 9,640 | 1.74 | – | 488.49 pg/mL | Adenoma |
| 19, (22) | M | 64 | 170/100 | 3,874 | 1.17 | – | – | N |
| 20, (23) | F | 36 | 152/108 | 11,000 | 2.2 | 0.2 ng/mL/h | 17.4 pg/dL | N |
| 21, (24) | F | 49 | – | 1,753 | 1.8 | 1.06 pg/mL | 648.9 pg/mL | Adenoma |
| 22, (25) | M | 55 | 138/68 | 15,760 | 1.4 | 0.1 ng/mL/h | 26.6 ng/dL | Adenoma |
| 23, (26) | F | 42 | 166/108 | 21,000 | 1.3 | – | 96.6 ng/dL | Adenoma |
| 24, (27) | F | 45 | 143/80 | 4,907 | 1.38 | – | 639.38 ng/L | Adenoma |
| 25, (27) | F | 44 | – | 8,531 | 1.98 | 0.84 ng/mL/h | 449.7 ng/L | Adenoma |
| 26, (28) | M | 73 | 140/80 | 7,463 | 1.6 | 0.7 ng/mL/h | 498 pg/mL | Hyperplasia |
| 27, (29) | F | 52 | – | 10,615 | 1.9 | 0.5 pg/mL | 295 pg/mL | Adenoma |
| 28, (30) | M | 49 | 150/100 | 12,030 | 2.4 | 0.3 ng/mL/h | 90 ng/dL | N |
| 29, (31) | F | 27 | 140/110 | 9,590 | 1.89 | – | 295.3 pmol/L | Adenoma |
| 30, (32) | M | 45 | 170/105 | 6,400 | 1.4 | 0.2 μg/L/h | 1 015 ng/L | Adenoma |
| 31, (33) | F | 26 | 180/150 | 5,456 | 2.8 | 0.45 μg/L/h | 438.5 pmol/L | Adenoma |
| 32, (34) | F | 40 | 130/80 | 12,085 | 1.66 | 0.2 ng/mL/h | 28.67 ng/dL | N |
PA, hyperaldosteronism; RM, rhabdomyolysis; F, female; M, male; CK, creatine kinase; K, potassium; Ald, aldosterone; N, no results provided.
The relationship between thyroid hormones and RM: thyroid hormones increase Na/K ATPase activity in skeletal muscle, liver, and kidney through transcriptional and post-transcriptional mechanisms, inducing the flow of potassium into the intracellular space. Our patient was admitted to the hospital with myasthenia as the main manifestation. Electrolyte examination indicated severe hypokalemia and significantly elevated kinase profile, and the patient was diagnosed with RM. In addition, comprehensive examination confirmed hypertension, which caused corresponding changes in the fundus artery, indicating that the course of hypertension in the patient was not short, that is, the course of PA was not short, and the tolerance of most patients with PA to low potassium increases with prolongation of the disease course. However, the patient suddenly exhibited symptoms of muscle soreness and weakness that developed into RM before seeing a doctor. We speculated that the patient’s metabolic thyroid hormone disorder increased the severity and accelerated the development of hypokalemia. The cause of thyrotoxicosis hypokalemic periodic paralysis is related to disordered potassium metabolism. When the attack occurs, the blood potassium is often reduced, and the paralysis is relieved after potassium supplementation. Because thyroid hormone increases the activity of enzymes in skeletal muscle cells, it accelerates the transfer of potassium from the extracellular to the intracellular space. In addition, patients with thyrotoxicosis have abnormal calcium levels. Calcium ions transfer from the extracellular to the intracellular space, which can affect the excitation-contraction coupling of the muscles, resulting in periodic muscle paralysis.
Through literature review, it was found that reports of RM caused by single thyrotoxicosis were rare. More interestingly, the related literature was searched in CNKI database, VIP database, Wanfang Database and PubMed with the keywords of “thyroid” and “rhabdomyolysis” from 2017 to now. We found that most reported RM related to thyroid dysfunction was hypothyroidism rather than thyrotoxicosis. Specifically, we excluded RM caused by other factors such as drugs, trauma, infection, fatigue and so on. Since 2017, 140 articles and 17 cases met the requirements, and the summary is shown in Table 5. Previous reports have suggested that primary hypothyroidism is associated with myopathy, myositis and elevated muscle enzymes, which is a clear risk factor for RM. It has been previously reported that primary hypothyroidism is associated with myopathy, myositis, and elevated muscle enzyme, which is a clear risk factor for RM syndrome. Hashimoto’s thyroiditis as the etiology of RM syndrome has also been reported (44). However, the exact cause of the disease remains unclear, which may be related to the fact that insufficient thyroxine inhibits the decomposition of liver sugars, mitochondrial oxidation, and the conversion of triacylglycerol into energy, which eventually leads to muscle injury and even RM under oxidative stress conditions (45). Hypothyroidism is associated with a good prognosis for RM syndrome, and RM and acute renal insufficiency can be quickly reversed with thyroid hormone replacement (41,46). Reports of thyrotoxicosis-related RM are rare. The prognosis of the case of thyrotoxicosis-induced RM reported in this study was good; however, whether it is representative or not remains unclear and should be summarized in more cases.
Table 5
| Number | Gender | Age (years) | TSH | FT3 | FT4 | TPO-Ab | Tg-Ab | K | Myalgia | Myasthenia | Brown urine | RAIU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 36 | 0.01 mIU/mL | 2.24 nmol/L | 34.2 pmol/L | 34 IU/mL | <15 IU/mL | 2.59 mmol/L | Y | Y | N | ↓ |
| 1, (35) | M | 49 | 49.60 mU/L | 1.79 pmol/L | 0.08 pmol/L | 331.90 U/mL | 2,503.0 U/mL | – | Y | Y | N | ↓ |
| 2, (17) | F | 62 | >100 μU/mL | – | – | – | – | – | N | N | N | ↓ |
| 3, (17) | F | 43 | >100 μU/mL | – | – | – | – | – | Y | Y | N | ↓ |
| 4, (17) | F | 53 | >100 μU/mL | – | – | – | – | – | Y | N | N | ↓ |
| 5, (17) | M | 49 | >100 μU/mL | – | – | – | – | – | Y | Y | N | ↓ |
| 6, (36) | M | 65 | 62.445 IU/L | 1.54 pmol/L | 5.15 pmol/L | 600 kU/L | 4,000 kU/L | 4 mmol/L | N | N | N | ↓ |
| 7, (37) | M | 62 | 150 mU/L | 0.64 ng/L | 0.2 ng/dL | >1,300 kU/L | >500 kU/L | – | N | Y | N | ↓ |
| 8, (37) | M | 54 | 65.326 mU/L | 1.53 ng/L | 0.47 ng/dL | >1,300 kU/L | >500 kU/L | – | Y | N | N | ↓ |
| 9, (37) | M | 86 | 93 mU/L | 0.9 ng/L | 0.48 ng/dL | 144.93 kU/L | 23.6 kU/L | – | Y | N | Y | ↓ |
| 10, (37) | M | 68 | 150 mU/L | 0.8 ng/L | 0.54 ng/dL | >1,300 kU/L | >500 kU/L | – | Y | Y | Y | ↓ |
| 11, (37) | M | 52 | 29.683 mU/L | <0.2 ng/L | 0.1 ng/dL | 183.28 kU/L | 48 kU/L | – | N | Y | N | ↓ |
| 12, (38) | F | 58 | 100 μIU/mL | 0.26 pg/mL | 0.06 ng/dL | 393.7 IU/mL | 4,000 IU/mL | – | Y | Y | N | ↓ |
| 13, (39) | F | 36 | 1.581 μIU/mL | 1.7 pmol/L | 3.72 pmol/L | – | – | – | Y | Y | Y | – |
| 14, (40) | M | 66 | 145.6 mIU/L | 0.06 pg/mL | 2.78 pmol/L | 661 IU/mL | – | – | Y | Y | Y | – |
| 15, (41) | M | 33 | 145.7 μIU/mL | – | 117.13 pmol/L | – | – | 4.2 mmol/L | Y | Y | N | – |
| 16, (42) | M | 62 | – | – | 2.94 ng/dL | – | – | – | Y | Y | N | – |
| 17, (43) | M | 29 | >114 µIU/mL | 0.3 pg/mL | 1.7 pg/mL | – | – | – | Y | Y | N | – |
RM, rhabdomyolysis; F, female; M, male; TSH, thyroid stimulating hormone; FT3, free triiodothyronine; FT4, free tetraiodothyronine; TPO-Ab, thyroid peroxidase antibody; Tg-Ab, anti-thyroglobulin antibody; K, potassium; Y, yes; N, no; RAIU, radioactive iodine uptake.
In addition to this, hypokalemia is uncommon as a causative agent of RM. It has been reported in the literature that hypokalemia accounts for only a small proportion of the etiology of RM. In one report (15), a rare case of RM was caused by severe hypokalemia, which in turn was caused by PA. Furthermore, it was reported (15) that RM was detected early as a serious complication of hypokalemia, and other causes of hypokalemia were gradually excluded until the final diagnosis of familial hypokalemic periodic paralysis was reached.
Another unique feature of this patient was that his thyrotoxicosis was not caused by classic Graves’ disease, but by non-classic thyroiditis. These entities are difficult to diagnose in the clinical setting because limb weakness in young men combined with abnormal thyroid function and decreased blood potassium is easily misdiagnosed as “peripheral paralysis of hyperthyroidism and hypokalemia”. However, we assessed thyroid-related antibodies in a timely manner and found that the antibody levels were not high. In addition, we assessed the iodine uptake rate, which indicated low iodine uptake. These findings made the diagnosis of thyroiditis clear. At the same time, we initiated further screening and sought the true cause of the hypokalemia. Moreover, the correct diagnosis of aldosteronism laid a foundation for subsequent assessments. In conclusion, improved understanding and familiarity with the core clinical characteristics of the disease, especially rare complications or exceptions of comorbidities, can lead to timely diagnosis and treatment, improving the rate of correct diagnosis to improve the care of patients.
Conclusions
Considering the high prevalence and harmfulness of hyperaldosteronism in today’s medicine, it is important for physicians to diagnose and treat them in a timely and correct manner, and early detection and treatment is very beneficial for patients. For doctors, we should probably discover areas that don’t receive much attention in the conventional medical treatment process, and enrich the diagnosis experience, so as to propose a more complete treatment idea.
Acknowledgments
The authors would like to thank the patient for his involvement in this study, who also allowed the authors to publish the case report and use the images taken during his hospital admission. The authors also want to thank the Yiji-to-Edit Rewriters for their help with the grammar and punctuation of this article.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-103/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-103/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-103/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101:1889-916. [Crossref] [PubMed]
- Montori VM, Schwartz GL, Chapman AB, et al. Validity of the aldosterone-renin ratio used to screen for primary aldosteronism. Mayo Clin Proc 2001;76:877-82. [Crossref] [PubMed]
- Buffolo F, Tetti M, Mulatero P, et al. Aldosterone as a Mediator of Cardiovascular Damage. Hypertension 2022;79:1899-911. [Crossref] [PubMed]
- Mulatero P, Monticone S, Bertello C, et al. Evaluation of primary aldosteronism. Curr Opin Endocrinol Diabetes Obes 2010;17:188-93. [Crossref] [PubMed]
- Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3266-81. [Crossref] [PubMed]
- Lee JH, Kim E, Chon S. Hypokalemia-Induced Rhabdomyolysis by Primary Aldosteronism Coexistent With Sporadic Inclusion Body Myositis. Ann Rehabil Med 2015;39:826-32. [Crossref] [PubMed]
- Loh KC, Koay ES, Khaw MC, et al. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. J Clin Endocrinol Metab 2000;85:2854-9. [Crossref] [PubMed]
- Qin H, Gong L, Wang Y, et al. Two cases of primary aldosteronism combined with rhabdomyolysis and review of the literature. Journal of Chongqing Medical University 2018;43:763-7.
- Han R, Jiang X. Hypokalemia-induced rhabdomyolysis as the first symptom of primary aldosteronism: a case report and literature review. Ann Palliat Med 2022;11:2778-84. [Crossref] [PubMed]
- Chen CT, Wang YC, Lin CM. Hypokalemia-Induced Rhabdomyolysis Caused by Adrenal Tumor-Related Primary Aldosteronism: A Report of 2 Cases. Am J Case Rep 2021;22:e929758. [Crossref] [PubMed]
- Pecnik P, Müller P, Vrabel S, et al. Two cases of hypokalaemic rhabdomyolysis: same but different. BMJ Case Rep 2018;2018:bcr2017223609. [Crossref] [PubMed]
- Pintavorn P, Munie S. A Case Report of Recurrent Hypokalemia During Pregnancies Associated With Nonaldosterone-Mediated Renal Potassium Loss. Can J Kidney Health Dis 2021;8:20543581211017424. [Crossref] [PubMed]
- Maung AC, Kerwen AK, Ching LP. Hypokalaemic rhabdomyolysis as initial presentation of primary aldosteronism. J R Coll Physicians Edinb 2021;51:149-52. [Crossref] [PubMed]
- Sirkeci O, Sirkeci EE, Kucukciloglu Y. Severe Hypokalemia and Rhabdomyolysis Caused by Conn Syndrome. Clin Ter 2021;172:407-9. [Crossref] [PubMed]
- Jung YL, Kang JY. Rhabdomyolysis following severe hypokalemia caused by familial hypokalemic periodic paralysis. World J Clin Cases 2017;5:56-60. [Crossref] [PubMed]
- Gao PL, Yang Z. A case of primary aldosteronism combined with rhabdomyolysis syndrome and review of the literature. Journal of Shanxi Medical University 2018;49:439-41.
- Gao C, Yuan J, Li Fan, et al. Clinical analysis of rhabdomyolysis syndrome complicated by endocrine diseases. Journal of Xi'an Jiaotong University 2020;41:919-22. (Medical Edition).
- Kang J, Su PX, Deng BM, et al. A case of adrenal adenoma-type primary aldosteronism complicated by rhabdomyolysis. Journal of Diagnostics Concepts & Practice 2019;18:583-4.
- Zhang H, Zou Y, Zhang Y, et al. A case of primary aldosteronism complicated by rhabdomyolysis. Chinese Circulation Journal 2006;21:477.
- Chen L, Han XM, Liu GX, et al. Primary aldosteronism combined with hypokalemic rhabdomyolysis: a case report. Chinese Journal of Urology 2013;34:719.
- Wang Y, Chen J, Yang K, et al. Primary aldosteronism causing severe hypokalemia and rhabdomyolysis in a case. Journal of Critical Care in Internal Medicine 2012;18:383-4.
- Ruan ZF, He WQ. A case of rhabdomyolysis due to severe hypokalemia. Chinese Journal of Difficult and Complicated Cases 2012;11:474.
- Petidis K, Douma S, Aslanidis S, et al. Hypertension associated with rhabdomyolysis. J Clin Hypertens (Greenwich) 2007;9:60-2. [Crossref] [PubMed]
- Tsai WT, Chen YL, Yang WS, et al. Primary aldosteronism associated with severe hypokalemic rhabdomyolysis. Hormones (Athens) 2012;11:505-6. [Crossref] [PubMed]
- Goto A, Takahashi Y, Kishimoto M, et al. Primary aldosteronism associated with severe rhabdomyolysis due to profound hypokalemia. Intern Med 2009;48:219-23. [Crossref] [PubMed]
- Cooray MS, Bulugahapitiya US, Peiris DN. Rhabdomyolysis: A rare presentation of aldosterone-producing adenoma. Indian J Endocrinol Metab 2013;17:S237-9. [Crossref] [PubMed]
- Wen Z, Chuanwei L, Chunyu Z, et al. Rhabdomyolysis presenting with severe hypokalemia in hypertensive patients: a case series. BMC Res Notes 2013;6:155. [Crossref] [PubMed]
- Kotsaftis P, Savopoulos C, Agapakis D, et al. Hypokalemia induced myopathy as first manifestation of primary hyperaldosteronism - an elderly patient with unilateral adrenal hyperplasia: a case report. Cases J 2009;2:6813. [Crossref] [PubMed]
- Etgen T, Gräbert C. Tetraparesis with hypertensive crisis: hypokalemic rhabdomyolysis in primary hyperaldosteronism. Nervenarzt 2009;80:717-9. [Crossref] [PubMed]
- Dominic JA, Koch M, Guthrie GP Jr, et al. Primary aldosteronism presenting as myoglobinuric acute renal failure. Arch Intern Med 1978;138:1433-4. [Crossref] [PubMed]
- Hou Q, Wang J, Pei J, et al. A case of primary aldosteronism complicated by severe skeletal muscle damage. Chinese Journal of Internal Medicine 2004;68. [PubMed]
- Zhang YL, Jiang YN, Liu Y. A case of severe hypokalemia causing multiple organ damage in primary aldosteronism. Chinese Journal of Hypertension 2015;23:695-7.
- Pan XL, Zhao XW. A case of primary aldosteronism causing hypokalemia and elevated muscle enzymes. Chinese Journal of Hypertension 2012;20:595-6.
- Zavatto A, Concistrè A, Marinelli C, et al. Hypokalemic rhabdomyolysis: a rare manifestation of primary aldosteronism. Eur Rev Med Pharmacol Sci 2015;19:3910-6. [PubMed]
- He G. A case of rhabdomyolysis syndrome secondary to hypothyroidism. Chinese Journal of Rural Medicine and Pharmacy 2021;28:49+58.
- Shi YP, Zhu N, Rong S. A case of hypothyroid myopathy complicated by rhabdomyolysis resulting in acute kidney injury. Journal of New Medicine 2019;50:793-6.
- Zhang J, Guo LX. Clinical analysis of 12 cases of rhabdomyolysis syndrome complicated by endocrine diseases. Chinese Journal of Practical Internal Medicine 2016;36:903-5.
- Xu Z, Tu XW, Yu J. Report of a case of hypothyroid myopathy combined with rhabdomyolysis first diagnosed with nephritis. Beijing Medical Journal 2017;39:1055+1058.
- Yu HM, Jiao FL. A case of rhabdomyolysis caused by fenofibrate. Journal of Hubei University of Medicine 2017;36:170-1.
- Ren L, Wei C, Wei F, et al. A case report of rhabdomyolysis and osteofascial compartment syndrome in a patient with hypothyroidism and diabetes. BMC Endocr Disord 2021;21:212. [Crossref] [PubMed]
- Naz A, Issa M. Rhabdomyolysis and acute renal impairment in a patient with hypothyroidism: a case report. Case Rep Med 2014;2014:139170. [Crossref] [PubMed]
- Silverman MB, Wray J, Bridwell RE, et al. Thyroid Storm, Rhabdomyolysis, and Pulmonary Embolism: An Unusual Triad Case Report. Clin Pract Cases Emerg Med 2020;4:540-3. [Crossref] [PubMed]
- Jobé J, Corman V, Fumal A, et al. Rhabdomyolysis and hypothyroidism. Rev Med Liege 2007;62:484-6. [PubMed]
- Nikolaidou C, Gouridou E, Ilonidis G, et al. Acute renal dysfunction in a patient presenting with rhabdomyolysis due to Hypothyroidism attributed to Hashimoto's Disease. Hippokratia 2010;14:281-3. [PubMed]
- Shaikhouni S, Yessayan L. Management of Acute Kidney Injury/Renal Replacement Therapy in the Intensive Care Unit. Surg Clin North Am 2022;102:181-98. [Crossref] [PubMed]
- Cai Y, Tang L. Rare acute kidney injury secondary to hypothyroidism-induced rhabdomyolysis. Yonsei Med J 2013;54:172-6. [Crossref] [PubMed]
Cite this article as: Zhang Y, Ni W, Wang W. A case of primary aldosteronism with rhabdomyolysis in which the first symptoms were thyrotoxicosis and peripheral paralysis and literature review. AME Case Rep 2024;8:20.