Balloon-mounted covered stent as endovascular management of a traumatic cervical internal carotid artery pseudoaneurysm in a 23-year-old: a case report
Highlight box
Key findings
• We report one of the first cases in the literature regarding the management via PK Papyrus (Biotronik, Lake Oswego, Oregon, USA) balloon-mounted covered stent of a 23-year-old male with an enlarging cervical carotid artery pseudoaneurysm and progressive internal carotid artery stenosis.
What is known and what is new?
• Traumatic pseudoaneurysms have been successfully treated with open surgery and stents.
• This case is one of the first traumatic cervical internal carotid artery pseudoaneurysms to be treated with a PK Papyrus balloon-mounted covered stent.
What is the implication, and what should change now?
• These positive outcomes support the use of a balloon-mounted covered stent as a safe and feasible modality with high technical success for endovascular management of pseudoaneurysm. Further testing of this type of stent is warranted on a greater number of patients.
Introduction
Cervical internal carotid artery (cICA) pseudoaneurysms can be a primary cause of stroke in young adults (1,2). The most common etiology is trauma (3). Complications include rupture and thromboembolic events (2,4), which require treatment in select cases. Therapeutic lines include carotid occlusion, surgical ligation, surgical bypass, flow diverting embolization device (2), and stent-assisted coiling (5,6). Surgical options are challenging and more invasive. Stent coiling is associated with higher recurrence rates, and flow diversion does not provide immediate protection. Endovascular covered stents (7,8) are a recent treatment modality that could be offered for cICA pseudoaneurysms which preserve the carotid artery and provide immediate aneurysm occlusion. Limited data has reported the feasibility of this option (5-7). Here we describe a case of a traumatic cICA pseudoaneurysm treated with a PK Papyrus balloon-mounted covered stent. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-56/rc).
Case presentation
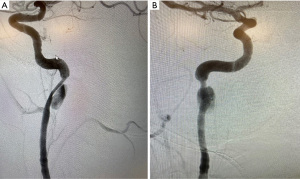
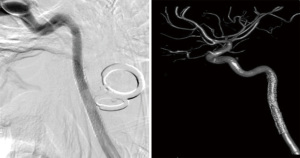
A 23-year-old male patient was referred to our center for an enlarging right cICA pseudoaneurysm with severe luminal stenosis of the parent vessel. The patient’s history was negative for headache, visual disturbance, weakness, and relevant family history, but positive for cerebral contusions and an un-helmeted motorcycle accident without loss of consciousness 3 months prior to referral. The patient was on a daily regimen of aspirin and clopidogrel. A diagnostic computed tomography angiogram (CTA) of the neck at an outside hospital during the initial presentation was concerning for a right cICA dissection with associated pseudoaneurysm and parent vessel luminal stenosis. A diagnostic catheter angiography after referral was ordered, which revealed an enlarging pseudoaneurysm of 1.7 cm in the greatest dimension and luminal stenosis equal to at least 60% of the normal ICA. The dissection involved the distal cICA, extending up to the proximal petrous segment of the ICA. Cerebral angiogram confirmed these diagnostic catheter angiography findings (Figure 1A,1B).
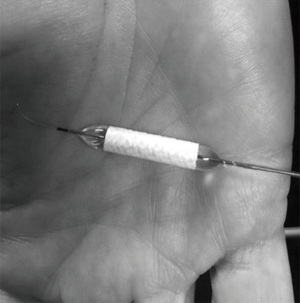
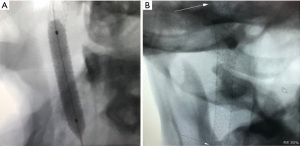
Based on clinical judgment and imaging analysis, we decided that the best option to seal the aneurysm was a PK Papyrus 5×26 balloon-mounted covered stent (Biotronik, Lake Oswego, Oregon, USA) (Figure 2). Under general anesthesia, a 6-French (Fr) Berenstein diagnostic catheter (Merit Medical, South Jordan, Utah, USA) inside a Benchmark catheter was advanced over an angled 0.0035 guidewire and introduced through a sheath into the right common femoral artery. It was thereafter navigated into the left subclavian to the left vertebral artery and then maneuvered into the left internal carotid artery, where it remained in position. We performed a balloon occlusion test first, in case we could not repair the vessel with a stent. For this test, we introduced a TransForm 7×7 mm compliant balloon (Stryker Neurovascular, Fermont, California, USA) over Transend ES 0.014 microwire (Stryker Neurovascular, Fermont, California, USA). The balloon was navigated over the microwire into the straight segment of the right ICA and positioned proximal to the pseudoaneurysm. The patient passed the balloon test occlusion with less than one second of venous delay. The PK Papyrus 5×26 balloon-mounted covered stent was in position and deployed across the distal cICA (Figure 3A,3B), providing appropriate coverage of the pseudoaneurysm neck. The balloon was then inflated to near maximal pressure to allow a better position of the stent across the distal ICA (expansion to 5.5 mm). No distal protection device was utilized.
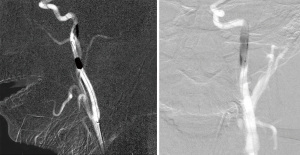
A follow-up angiogram showed no residual filling of the pseudoaneurysm, but there was some contrast stagnation just proximal to the stent, which is consistent with a residual dissection flap; therefore, we deployed another PK Papyrus 5×26 balloon-mounted covered stent, providing some overlap at the proximal end of the stent. An angiogram following this subsequent deployment demonstrated complete reconstruction of the cICA with no residual evidence of pseudoaneurysm or dissection flap. There were no residual in-stent stenosis or vessel stenosis (Figure 4). No procedural complications were experienced. The patient did well postoperatively and was discharged the day after surgery. We planned to put the patient on aspirin 325 mg daily for life and clopidogrel 75 mg for 6 months. At the 6-month follow-up, the patient denied any symptoms, and there was no evidence of pseudoaneurysm or dissection on the angiogram performed at that time (Figure 5). Long-term follow-up for this patient included an annual CTA.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Pseudoaneurysm is a rare pathology, accountable for 5.6% of all carotid aneurysms (9) and less than 1% of all arterial aneurysms (10). Literature is scarce regarding the use of covered stents in treating cerebrovascular conditions (11). Lu et al. used covered stents in 6% of their series of 32 carotid-cavernous fistula patients (12). The reported use of the covered stents in cICA pseudoaneurysm is even less than that. Pinzón et al. (7) and Wang et al. (5) have performed procedures using covered stents in pediatric populations. Our study highlights initial experiences with covered stents for neuroendovascular use, with only two other similar cases reported in the neuroendovascular literature, both of which investigate the safety and efficacy of covered stents in adults with distal cICA pseudoaneurysm (13,14).
The PK Papyrus-covered coronary stent used in our study is a humanitarian device exemption (HDE) approved by the United State Food and Drug Administration (FDA) for the treatment of acute perforations of coronary arteries with a diameter of 2.5–5.0 mm (15,16). The potential benefits of this stent include a quicker deployment time, thinner build, and better maneuverability than other covered stents, which contribute to technical success and minimize potential complications (15,17). The stent’s covering is distinctive as it leads to the immediate obliteration of the pseudo or traumatic aneurysm with 100% coverage as opposed to flow diverting stents which provide 30–35% and often take months to achieve full occlusion.
Additionally, the balloon-mounting of the PK Papyrus-covered stent is advantageous in that it has a greater radial force and can treat frequently associated parent vessel stenosis. It requires a smaller introductory sheath and carries less risk for embolization by pre-mounting the stent on the catheter. Notably, it is available in larger sizes than the current flow diverting stents, with a maximum expansion diameter of 5.63 mm (15). If conventional pipeline stents, of which the largest diameter is only 5 mm, are used instead of the PK Papyrus-covered stent, there is a higher chance that the stent will be floating and not oppose the vessel, increasing the risk of stroke in the patient. The PK Papyrus stent also has advantages over other covered stents. The smaller size of the PK Papyrus stent is significantly thinner and more trackable than the traditionally covered Viabahn stents. Unlike the PK Papyrus stent, Viabahn-covered stents are too large and thick to track past tortuous distal cervical anatomy, the petrous segment of the internal carotid artery, or the V2 segment of the vertebral artery. Also, notably, a distal protection device was not utilized in this presented case and is not needed with the PK Papyrus stent unless it is used for an atherosclerotic lesion. Overall, this balloon-mounted covered stent could be a promising modality when conventional microsurgical clipping or endovascular options, including flow diverting stents, cannot treat complex traumatic or pseudoaneurysms with a broad base and frequently associated stenosis of the parent vessel.
Contrarily, it is important to highlight the disadvantages of the PK Papyrus balloon-mounted covered stent. Due to the stent being covered, it may be slightly precluded due to stiffness and difficult navigation with the risk of perforator occlusion in intracranial pathology. Regarding the balloon-mounted aspect of the stent, although it is an optimal device for straight segments such as the cICA, it is less so for lesions located from and distal to the petrous segment. This discrepancy is due to the anatomy of the latter segments, which are surrounded by skull base and may carry difficulty in navigating and inflating the balloon. However, it can still be effectively used to pass through torturous distal cervical anatomy as well as the petrous segments of the ICA and V2 segment of the vertebral artery.
It is important to note limitations of our study; it is a case report of a singular patient. Further testing of balloon-mounted covered stents on a substantial number of patients is warranted to gather statistically sound results for further analysis into technical success and outcomes and to corroborate our claims.
Conclusions
Our case is the third to our knowledge reported in the neuroendovascular literature to use the PK Papyrus-covered stent for cICA pseudoaneurysm. A balloon-mounted covered stent is a safe and feasible reconstructive modality for cICA pseudoaneurysm. Further use of this stent is encouraged to explore the safety and efficacy of the device.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-56/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-56/coif). R.M.S.’s research is supported by the Neurosurgery Research & Education Foundation (NREF), Joe Niekro Foundation, Brain Aneurysm Foundation, Bee Foundation, and by National Institute of Health (NIH) (No. R01NS111119-01A1) and (No. UL1TR002736 and KL2TR002737) through the Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. R.M.S. has an unrestricted research grant from Medtronic and has consulting and teaching agreements with Penumbra, Abbott, Medtronic, Balt, InNeuroCo, Cerenovus, Naglreiter and Optimize Vascular. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery 2011;68:856-66; discussion 866. [Crossref] [PubMed]
- Akinduro OO, Gopal N, Hasan TF, et al. Pipeline Embolization Device for Treatment of Extracranial Internal Carotid Artery Pseudoaneurysms: A Multicenter Evaluation of Safety and Efficacy. Neurosurgery 2020;87:770-8. [Crossref] [PubMed]
- Spanos K, Karathanos C, Stamoulis K, et al. Endovascular treatment of traumatic internal carotid artery pseudoaneurysm. Injury 2016;47:307-12. [Crossref] [PubMed]
- Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 1999;47:845-53. [Crossref] [PubMed]
- Wang A, Santarelli JG, Stiefel MF. Traumatic cervical internal carotid artery pseudoaneurysm in a child refractory to initial endovascular treatment: case report and technical considerations. Childs Nerv Syst 2016;32:2459-64. [Crossref] [PubMed]
- Bannazadeh M, Sattari AR, Skripochnik E, et al. Endovascular repair of infected carotid pseudoaneurysm: A case report. Int J Surg Case Rep 2020;72:163-5. [Crossref] [PubMed]
- Pinzón M, Lobelo NO, Rodríguez MC, et al. Endovascular management of iatrogenic cervical internal carotid artery pseudoaneurysm in a 9-year-old child: Case report and literature review. Int J Pediatr Otorhinolaryngol 2017;95:29-33. [Crossref] [PubMed]
- Requejo F, Sierre S, Lipsich J, et al. Endovascular treatment of post-pharyngitis internal carotid artery pseudoaneurysm with a covered stent in a child: a case report. Childs Nerv Syst 2013;29:1369-73. [Crossref] [PubMed]
- da Silva PS, Waisberg DR. Internal carotid artery pseudoaneurysm with life-threatening epistaxis as a complication of deep neck space infection. Pediatr Emerg Care 2011;27:422-4. [Crossref] [PubMed]
- Sharma RK, Asiri AM, Yamada Y, et al. Extracranial Internal Carotid Artery Aneurysm - Challenges in the Management: A Case Report and Review Literature. Asian J Neurosurg 2019;14:970-4. [Crossref] [PubMed]
- Ding D, Starke RM, Moriarty M, et al. Pericardium Covered Stent Graft for Endovascular Treatment of a Traumatic Carotid-cavernous Fistula. J Neurosci Rural Pract 2016;7:S137-8. [Crossref] [PubMed]
- Lu X, Hussain M, Ni L, et al. A comparison of different transarterial embolization techniques for direct carotid cavernous fistulas: a single center experience in 32 patients. J Vasc Interv Neurol 2014;7:35-47. [PubMed]
- Choudhri O, Heit J, Do HM. Endovascular reconstruction of enlarging traumatic internal carotid artery pseudoaneurysm. Neurosurg Focus 2014;37:1. [Crossref] [PubMed]
- Scavée V, De Wispelaere JF, Mormont E, et al. Pseudoaneurysm of the internal carotid artery: treatment with a covered stent. Cardiovasc Intervent Radiol 2001;24:283-5. [Crossref] [PubMed]
- PK Papyrus. (n.d.). PK Papyrus. [retrieved April 22, 2022]. Available online: https://pkpapyrus.com/
- Office of the Commissioner. (2018, September 14). FDA approves devices for the treatment of acute coronary artery perforations. U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-device-treatment-acute-coronary-artery-perforations#:%7E:text=The%20U.S.%20Food%20and%20Drug,this%20indication%20in%2017%20years
- Tahir H, Livesay J, Baljepally R, et al. Successful Rescue Intervention of Internal Mammary Artery Anastomotic Site Acute Graft Failure With Direct New Generation Covered Stenting. J Med Cases 2021;12:271-4. [Crossref] [PubMed]
Cite this article as: Abdelsalam A, Saini V, Eatz T, Silva MA, Luther EM, Bandes M, Thompson JW, Ramsay IA, Burks JD, Fountain HB, Starke RM. Balloon-mounted covered stent as endovascular management of a traumatic cervical internal carotid artery pseudoaneurysm in a 23-year-old: a case report. AME Case Rep 2024;8:17.