
Calcitonin-secreting neuroendocrine tumor of the larynx, a diagnostic challenge of a rare neoplasm: a case report and literature review
Highlight box
Key findings
• Diagnostic challenge of a rare aggressive cancer in a rare site of head and neck: a calcitonin-secreting laryngeal neuroendocrine tumor (NET).
What is known and what is new?
• Laryngeal NETs are extremely rare, and available data are limited on case reports: there are 7 described cases of calcitonin-secreting primary laryngeal NETs.
• This case report shows that the diagnosis of laryngeal NET is a medical and therapeutic challenge. Moreover, this manuscript highlights the possibility of promptly diagnosing a relapse of the disease by serum calcitonin dosing.
What is the implication, and what should change now?
• Further studies are needed to establish the serum calcitonin range values suggesting an early relapse and the correct timing of its dosing.
Introduction
Laryngeal neuroendocrine neoplasms (NENs) represent less than 1% of all malignancies originating from the larynx (1).
Laryngeal NENs are characterized by neuroendocrine differentiation (distinct from neural forms, such as paraganglioma) (2) and probably derived from Kulchitsky cells, neuroendocrine cells identified at the base and middle layer of the respiratory tract epithelium, mainly at ventricle and subglottis (3).
According to World Health Organization (WHO) 2017, NENs of head and neck are classified into three categories: well-differentiated tumor, or grade1 (G1, previously called “typical carcinoid”), accounting for approximately 5% of laryngeal NENs, which generally occur in the supraglottic region (4,5); moderately differentiated tumor, or G2 (formerly referred to as “atypical carcinoid”), the most frequent type, accounting for 54% of laryngeal NENs (5,6); poorly differentiated carcinoma, G3 neuroendocrine cancer (NEC), which is the second most common type of laryngeal NENs (48.6%), including two subtypes, small cells neuroendocrine cancer (SNEC) or small cell undifferentiated neuroendocrine carcinoma (SCUNC) (41.9%) and large cells neuroendocrine cancer (LCNEC) (6.7%) (5,7,8).
The incidence of laryngeal NENs is greater in males compared to females (3:1), with a history of smoking, usually in the fifth to seventh decade of life, and the most frequently involved site is the supraglottic area (9,10).
The symptoms at onset are generally related to mass growth (hoarseness, dysphonia, dysphagia, and pharyngodynia) and rarely associated to a paraneoplastic syndrome.
Laryngeal NENs are characterized by immunohistochemical neuroendocrine markers, such as chromogranin A (the most specific), synaptophysin (the most sensitive) and cytokeratins. However, calcitonin can be an additional neuroendocrine marker founded in laryngeal NENs (11). Although calcitonin immunostaining positivity is not an exceptional event, elevated serum calcitonin finding is extremely rare (3,10-15).
Moreover, the detection of elevated serum levels of calcitonin should be considered in the differential diagnosis with medullary thyroid carcinoma (MTC), even in the absence of suspicious lesions within the thyroid gland (16,17). Conversely, MTC rarely extends locally to the larynx, so differential diagnosis from primary laryngeal NENs is challenging (18).
The mainstay of well-differentiated, laryngeal NENs treatment is local excision in combination with elective neck dissection for moderately differentiated ones (10). On the other hand, poorly differentiated carcinomas (i.e., SCUNC and LCNEC), associated with poor prognosis, might benefit mostly from a combined chemotherapy and radiotherapy approach, depending on local extension and stage (9).
Here we report an atypical case of a G2 laryngeal NEN with elevated serum levels of calcitonin at diagnosis. The patient underwent radical surgical treatment, and was later treated with chemotherapy for disease relapse, based on the presence of histologically confirmed subcutaneous metastasis. Interestingly, serum calcitonin levels increased during relapse of the disease, suggesting a possible predictive role on disease recurrence.
A literature review of laryngeal NENs and their treatment options was performed and the predictive role of serum calcitonin levels on disease relapse in such patients was discussed. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-70/rc).
Case presentation
In May 2021, a 71-year-old female patient, never smoker, with negative familial history, presented with neck pain, radiating to the shoulder and left ear, associated to pharyngodynia and breakthrough pain lasting about a minute. At physical examination a mass of the tongue base occupying the left vallecula extended to the lingual face of the epiglottis and bilateral cervical lymphadenopathies were evident.
Contrast-enhanced computed tomography (CT) scan and 18F-fluoro-2-deoxy-D-glucose (FDG)-positron emission tomography (PET) confirmed the clinical findings and excluded distant metastases; an increased size of the nodular-looking thyroid gland suspected of primary thyroid cancer was further documented (Figure 1).
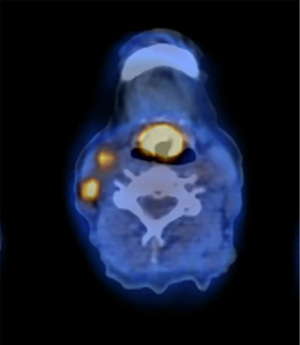
A transoral biopsy of the laryngeal lesion turned positive for a poorly differentiated neuroendocrine tumor expressing synaptophysin, neuron-specific enolase (NSE), chromogranin A, pan-cytokeratin including cytokeratin AE1-AE2 and focally calcitonin. Circulating NSE was 13.4 microg/L (normal level <12.5 microg/L), and basal calcitonin serum level was 237 pg/mL (normal level <11.5 pg/mL).
At ultrasound examination, the thyroid presented increased size and bilateral nodulations, the largest in the right lobe (20 mm), classified as TIR2 by fine needle aspiration cytology, according to the Italian consensus for the classification and reporting of thyroid cytology (19).
After multidisciplinary evaluation in April 2021, a supraglottic laryngectomy extended to the base of the tongue, bilateral selective neck dissection (level II–IV) and total thyroidectomy were performed.
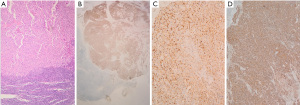
The pathology report of the resected tumor demonstrated a SCUNC (Figure 2A), G3, with Ki-67 index 60–70% and expression of neuroendocrine markers: synaptophysin (Figure 2B), NSE (Figure 2C), chromogranin A (Figure 2D) and broad spectrum cytokeratins. The primary tumor was excised with clear surgical margins and was staged as pT4a pN3b for the evidence of metastases in 25 out of 57 removed cervical lymph nodes with extracapsular invasion (3/14 positive lymph nodes at the third right level, 9/10 positive at the second right level, 4/14 positive at the third left level, 2/11 and 7/8 at the left level B and A, respectively). Thyroid gland histology revealed hyperplastic thyroid without evidence of cancer.
Genomic DNA from collected cancer tissue was extracted using a Qiagen DNA isolation kit (Qiagen Inc., Valencia, CA). Molecular analysis in Next Generation Sequencing with multigenic panel ONCOMINE and CUSTOM (Thermo Fisher Scientific, Bologna, Italy), revealed mutations in HRAS and MYC genes. Pyrosequencing analysis (Qiagen Inc., Valencia, CA, USA) showed a methylated MGMT gene.
After 40 days from surgery, 18F-FDG PET and a CT scan showed the appearance of a subcutaneous nodulation measuring 8 mm on the right middle axillary line (SUVmax =2.6, Figure 3A). On clinical examination, the axillary nodulation was fixed and firm. Pathology confirmed the presence of a metastasis from poorly differentiated carcinoma with neuroendocrine differentiation, compatible with the primary resected. The serum calcitonin after surgery was not measured due to the high predictability of raised levels in the presence of extracapsular disease. From September 2021 to November 2021, the patient was treated with four cycles of chemotherapy with carboplatin AUC5 on 1 day and etoposide 100 mg/m2 on days 1–3, every 3 weeks, with granulocyte colony-stimulating factor (G-CSF) as primary prophylaxis for febrile neutropenia. The treatment was well tolerated except for G1 diarrhea.
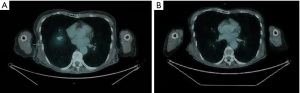
The subsequent CT scan, performed in December 2021, showed stable disease (SD) with dimensional stability of the single metastasis (8 mm in diameter) on the right middle axillary, while the 18F-FDG PET revealed normalization of uptake (Figure 3B). The serum calcitonin level was 57.9 pg/mL.
In January 2022 the patient was evaluated for persistent cervicalgia and a phenytoin therapy was initiated in the suspect of a glossopharyngeal neuralgia. In March 2022, 18F-FDG PET showed a locoregional recurrence of disease, due to appearance of a pathological radiotracer uptake at the right lateral cervical area (II level, SUVmax =7) and at the right side of the parapharyngeal region (SUVmax =7). Interestingly, calcitonin levels raised up to 89.3 pg/mL (vs. 57.9 after 4 cycles of chemotherapy).
Furthermore, the patient had persistence of neck pain, radiating to the shoulder blade and left ear, associated to pharyngodynia. After a new multidisciplinary evaluation, surgery was excluded for the extent of the disease and, considering the optimal metabolic response obtained with the previous treatment, the patient was started on a rechallenge with carboplatin and etoposide chemotherapy, which was well tolerated except for G1 nausea. After two cycles of treatment, a new 18F-FDG PET-CT showed disease progression as increased size and radiotracer uptake at the right submandibular space and homolateral parapharyngeal space.
Because of the lack of radiological response to first line treatment to which high-grade neuroendocrine tumors are usually sensitive, pathology revision was performed and confirmed a diagnosis of moderately differentiated NEN G2, with a Ki-67 index of 22.6%.
Thus, the case was also discussed at the Neuroendocrine Tumor Board and, in the light of histology and staging of disease, a second-line chemotherapy with capecitabine 500 mg twice daily on day 1 to 14 and temozolomide 200 mg daily on day 10 to 14 was administered to the patient for 3 cycles, with good tolerance.
However, patient’s general conditions declined in concurrence with pneumonia making her ineligible to an antiblastic treatment and was transitioned to best supportive care alone. The patient died in August 2022 because of pulmonary sepsis.
During the entire clinical course, the patient was fully aware of her condition and actively asked to be informed for every treatment she received. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
To the best of our knowledge, we report the eighth case of calcitonin-secreting laryngeal NEN in the international literature. The peculiarity of this rare case is represented by the complexity of histological diagnosis and therapeutic management highlighting the importance of a multidisciplinary team involving dedicated oncologists, otolaryngologists and expert pathologist who need to work closely to exchange best practices related to the cancer patient’s cure.
The patient’s disease progressed after surgery of laryngeal cancer, for the appearance of subcutaneous metastases, which required platinum-based chemotherapy, obtaining an initial response. However, during the follow-up, after four cycles of a first line platinum-based chemotherapy, the serum levels of calcitonin showed a growing trend (89.3 vs. 57.9 pg/mL after chemotherapy), suggesting a radiological recurrence of the disease, as confirmed by the subsequent 18F-FDG PET. Interestingly, a further increase of serum level of calcitonin (121 pg/mL) was noticed at the second disease progression as confirmed by radiological exams, leading the patient to start a different line with capecitabine and temozolomide.
The diagnosis of laryngeal NENs is challenging considering the rarity of these tumors. In order to address this issue, NET-expert pathologists should assess samples or revise unclear cases.
Laryngeal NENs are rare and aggressive neoplasms representing the most common non-squamous tumors of this site. They may be misdiagnosed and treated as undifferentiated carcinoma, squamous cell carcinoma or thyroid medullary carcinoma. The differential diagnosis of laryngeal tumors has received increasing attention since the first report of a primary carcinoid of the larynx in 1969 by Goldman et al. (20) and a primary laryngeal small cell neuroendocrine carcinoma in 1972 (21).
A correct diagnosis could certainly spare some patients an unnecessary thyroid ablation and reach better prognosis with adequate treatments.
The several subtypes of NENs show a variety of different treatments, natural histories and outcomes. They occurred mainly in middle aged and elderly men, often with a history of smoking and the most frequent site of origin is the supraglottic larynx. The most common subtype is the atypical carcinoid, followed by the small cell neuroendocrine carcinoma, typical carcinoid and the large cell neuroendocrine carcinoma (22).
The typically clinical onset symptoms are not specific and generally related to mass growth (hoarseness, dysphonia, dysphagia, and pharyngodynia). The patients often present with neck lymph nodes metastases and with painful metastases to the skin or to the scalp (23). In rare cases, laryngeal NENs are associated with an aberrant paraneoplastic syndrome due to hormone overproduction. In literature there are few cases of calcitonin-secreting primary laryngeal NENs. The first case was reported in 1981 by Sweeney et al. (17), a middle-aged man affected by a locally advanced left arytenoid tumor with an elevated serum calcitonin, who was treated with surgery. In 1990, Smets et al. (24) reported the case of a middle-aged man with the diagnosis of a metastatic moderately differentiated neuroendocrine tumor of the larynx secreting calcitonin and somatostatin. Insabato et al. (25) described a case report of a calcitonin-secreting moderately differentiated NEN of 2 cm of the right arytenoid treated with a partial laryngectomy and adjuvant radiotherapy. After 36 months the patient presented in a hypercalcitoninemic state, that helped identify a local recurrence of the tumor.
LaBryer et al. (14) presented a case report of a primary laryngeal moderately differentiated NEN associated with increased level of serum calcitonin and subcutaneous lymph nodes and thyroid metastases. Chung et al. (3) analyzed six patients with moderate differentiated laryngeal NENs, of which one patient showed elevated serum calcitonin levels during follow-up laboratory tests. He developed painful skin nodules in the right arm and bone metastases.
Machens et al. (23) described the same phenomenon in a middle-aged patient previously affected by moderately differentiated laryngeal NEN treated with surgery. During the follow-up, elevated calcitonin levels were noticed on laboratory tests and on physical examination several subcutaneous nodules were palpable.
Feola et al. (16) reported the case of a middle-aged man affected from a moderately differentiated laryngeal neuroendocrine tumor with lateral cervical lymphadenopathies and painful cutaneous lesions associated with increased levels of serum calcitonin. A somatostatin analog therapy was performed (octreotide LAR, 30 mg every 28 days), with stable disease as best response. After 5 months, the disease progressed at 18F-FDG PET-CT, and a new painful cutaneous lesions occurred. Considering high levels of serum calcitonin, differential diagnosis between MTC and calcitonin-secreting laryngeal NEN was required. The patient performed a thyroid color Doppler ultrasound, nodule fine needle aspiration and subsequent total thyroidectomy associated to cervical lymphadenectomy and cutaneous metastases resection aimed to pain relief and tumor debulking. The histological examination revealed not a MTC; two of the five resected lymph nodes, left upper parathyroid and skin lesions were metastases of NEN G2, positive for calcitonin. After 2 months, new painful skin lesions occurred and a target therapy with everolimus 10 mg/day was started. After 6 months of therapy 18F-FDG PET-CT detected metabolic partial response. An improvement of patient’s quality of life was also reached. Table 1 summarizes the seven cases of calcitonin-secreting laryngeal NEN.
Table 1
| Author, year | Diagnosis | Site | Stage at diagnosis | Treatment | Outcome | New treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Sweeney et al., 1981 (17) | Ectopic MCT/carcinoid tumor + well-differentiated squamous cell carcinoma | Left arytenoid | Locally advanced | Surgery (laryngothyroidectomy ) | – | – | – |
| Smets et al., 1990 (24) | Moderately differentiated NEN (atypical carcinoid) | Laryngeal surface of the epiglottis | Locally advanced | Radio + chemotherapy → CR on T and left neck dissection → radiotherapy + chemotherapy | After 18 months local recurrence | Total laryngectomy | One year later skin and single cerebral metastases → excision → after few months recurrence of skin lesions → chemotherapy. Death within 3 years of diagnosis |
| Insabato et al., 1993 (25) | Moderately differentiated NEN | Right arytenoid | Locally advanced | Surgery (partial laryngectomy) + RT | After 36 months local recurrence | Surgery | Negative follow up |
| LaBryer et al., 2015 (14) | Moderately differentiated NEN | Right arytenoid | Metastatic (lymph nodes and thyroid metastases) | Surgery (laryngectomy, thyroidectomy, and neck dissection) + adjuvant RT | After 6 months subcutaneous lesions | No further treatment | |
| Chung et al., 2004 (3) | Moderate differentiated laryngeal NEN | Epiglottis | Locally advanced | Surgery (supraglottic laryngectomy with neck dissection) + adjuvant CT | Painful skin nodules in the right arm and bone metastases | – | Death 27 months after surgery |
| Machens et al., 1999 (23) | Moderately differentiated laryngeal NEN | Dorsal aspect of the right aryepiglottic fold | Locally advanced | Surgery | After 2 years local recurrence and subcutaneous prelaryngeal metastases | Surgery | Negative follow up |
| Feola et al., 2020 (16) | Moderately differentiated laryngeal neuroendocrine NEN | Supraglottic left emilarynx | Metastatic (neck lymphadenopathies and painful cutaneous lesions) | SSA and palliative surgery | After 5 months of SSA treatment PD | Everolimus | PR after 6 months |
MCT, medullary carcinoma of thyroid gland; NEN, neuroendocrine neoplasia; CR, complete response; T, refers to the size and extent of the main tumor in TNM classification; RT, radiotherapy; CT, chemotherapy; SSA, somatostatin analog; PD, progressive disease; TNM, tumor-node-metastasis.
Conclusions
This is the eighth case report of laryngeal NET, highlighting the challenge in pathological differential diagnosis and therapeutic strategies. The association with elevated serum calcitonin and the trend of this parameter during clinical progression suggest a role of this marker in the diagnosis and early identification of recurrent laryngeal NETs.
Acknowledgments
Funding: The work reported in this publication was funded by Nancy International LTD.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-70/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-70/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-70/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan E, Mody MD, Saba NF. Systemic therapy in non-conventional cancers of the larynx. Oral Oncol 2018;82:61-8. [Crossref] [PubMed]
- Strosberg C, Ferlito A, Triantafyllou A, et al. Update on Neuroendocrine Carcinomas of the Larynx. Am J Clin Pathol 2019;152:686-700. [Crossref] [PubMed]
- Chung JH, Lee SS, Shim YS, et al. A study of moderately differentiated neuroendocrine carcinomas of the larynx and an examination of non-neoplastic larynx tissue for neuroendocrine cells. Laryngoscope 2004;114:1264-70. [Crossref] [PubMed]
- Medina JE, Moran M, Goepfert H. Oat cell carcinoma of the larynx and Eaton-Lambert syndrome. Arch Otolaryngol 1984;110:123-6. [Crossref] [PubMed]
- Trotoux J, Glickmanas M, Sterkers O, et al. Schwartz-Bartter syndrome. Presentation of a sub-glottal small cell laryngeal carcinoma (author's transl). Ann Otolaryngol Chir Cervicofac 1979;96:349-58. [PubMed]
- Bishop JW, Osamura RY, Tsutsumi Y. Multiple hormone production in an oat cell carcinoma of the larynx. Acta Pathol Jpn 1985;35:915-23. [Crossref] [PubMed]
- Perez-Ordoñez B. Neuroendocrine Carcinomas of the Larynx and Head and Neck: Challenges in Classification and Grading. Head Neck Pathol 2018;12:1-8. [Crossref] [PubMed]
- Gale N, Poljak M, Zidar N. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: What is New in the 2017 WHO Blue Book for Tumours of the Hypopharynx, Larynx, Trachea and Parapharyngeal Space. Head Neck Pathol 2017;11:23-32.
- van der Laan TP, Plaat BE, van der Laan BF, et al. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: A meta-analysis of 436 reported cases. Head Neck 2015;37:707-15. [Crossref] [PubMed]
- Ferlito A, Rinaldo A, Bishop JA, et al. Paraneoplastic syndromes in patients with laryngeal neuroendocrine carcinomas: clinical manifestations and prognostic significance. Eur Arch Otorhinolaryngol 2016;273:533-6. [Crossref] [PubMed]
- Giannetta E, Gianfrilli D, Pozza C, et al. Extrathyroidal Calcitonin Secreting Tumors: Pancreatic Neuroendocrine Tumors in Patients With Multinodular Goiter: Two Case Reports. Medicine (Baltimore) 2016;95:e2419. [Crossref] [PubMed]
- Ferlito A, Shaha AR, Rinaldo A. Neuroendocrine neoplasms of the larynx: diagnosis, treatment and prognosis. ORL J Otorhinolaryngol Relat Spec 2002;64:108-13. [Crossref] [PubMed]
- Ferlito A, Silver CE, Bradford CR, et al. Neuroendocrine neoplasms of the larynx: an overview. Head Neck 2009;31:1634-46. [Crossref] [PubMed]
- LaBryer L, Sawh R, McLaurin C, et al. Calcitonin-Secreting Neuroendocrine Carcinoma of Larynx with Metastasis to Thyroid. Case Rep Endocrinol 2015;2015:606389. [Crossref] [PubMed]
- Schneider R, Waldmann J, Swaid Z, et al. Calcitonin-secreting pancreatic endocrine tumors: systematic analysis of a rare tumor entity. Pancreas 2011;40:213-21. [Crossref] [PubMed]
- Feola T, Puliani G, Sesti F, et al. Laryngeal Neuroendocrine Tumor With Elevated Serum Calcitonin: A Diagnostic and Therapeutic Challenge. Case Report and Review of Literature. Front Endocrinol (Lausanne) 2020;11:397. [Crossref] [PubMed]
- Sweeney EC, McDonnell L, O'Brien C. Medullary carcinoma of the thyroid presenting as tumours of the pharynx and larynx. Histopathology 1981;5:263-75. [Crossref] [PubMed]
- White J, Mohyeldin A, Schwartz A, et al. Medullary thyroid carcinoma presenting as a supraglottic mass. Ear Nose Throat J 2014;93:E40-2. [Crossref] [PubMed]
- Nardi F, Basolo F, Crescenzi A, et al. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest 2014;37:593-9. [Crossref] [PubMed]
- Goldman NC, Hood CI, Singleton GT. Carcinoid of the larynx. Arch Otolaryngol 1969;90:64-7. [Crossref] [PubMed]
- Olofsson J, Van Nostrand AW. Anaplastic small cell carcinoma of larynx. Case report. Ann Otol Rhinol Laryngol 1972;81:284-7. [Crossref] [PubMed]
- Devaney KO, Ferlito A, Rinaldo A. Neuroendocrine carcinomas of the larynx: what do the different histologic types really mean? Eur Arch Otorhinolaryngol 2010;267:1323-5. [Crossref] [PubMed]
- Machens A, Holzhausen HJ, Dralle H. Minimally invasive surgery for recurrent neuroendocrine carcinoma of the supraglottic larynx. Eur Arch Otorhinolaryngol 1999;256:242-6. [Crossref] [PubMed]
- Smets G, Warson F, Dehou MF, et al. Metastasizing neuroendocrine carcinoma of the larynx with calcitonin and somatostatin secretion and CEA production, resembling medullary thyroid carcinoma. Virchows Arch A Pathol Anat Histopathol 1990;416:539-43. [Crossref] [PubMed]
- Insabato L, De Rosa G, Terracciano LM, et al. A calcitonin-producing neuroendocrine tumor of the larynx: a case report. Tumori 1993;79:227-30. [Crossref] [PubMed]
Cite this article as: Filippini DM, Abeshi A, Tober N, Marchese PV, Andrini E, Lamberti G, Agosti R, Molinari G, Fermi M, Presutti L. Calcitonin-secreting neuroendocrine tumor of the larynx, a diagnostic challenge of a rare neoplasm: a case report and literature review. AME Case Rep 2024;8:21.


