
Management of a decade-old recurrent chylothorax with breast fistulization: a case report
Highlight box
Key findings
• A decade-old chronic chylothorax fistulized with the breast to produce significant swelling, requiring a multidisciplinary approach to treatment when conservative measures failed.
What is known and what is new?
• Chylothorax management remains poorly studied, with escalating invasiveness corresponding to the leak persistence.
• This manuscript demonstrates the prognosis of a long-standing, undertreated chylothorax and the patient’s waning ability to compensate, despite medication and procedural adjuncts.
What is the implication, and what should change now?
• A literature review yielded sparse similarities between our case and others, highlight the uniqueness of our case. Few reports highlight the ability of the lymphatic system to fistulize and the difficulty in managing leaks to such a degree. Chylothorax management remains understudied with few high-quality trials guiding treatment.
Introduction
Every day, the thoracic duct is responsible for transporting 2.4 L of chyle, a triglyceride-rich fluid containing chylomicrons, lymph, and white blood cells (1,2). Any disruption of flow allows for excretion into the pleural space, causing a chylothorax. Common etiologies are subdivided by their mechanism, either traumatic (penetrating or blunt injury, forceful emesis, surgery, radiation, etc.) or non-traumatic (malignancy, tuberculosis, superior vena cava syndrome, etc.) (3). Considering the symptom-overlap with other intrathoracic pathology (dyspnea, fatigue, exercise intolerance), imaging and pleural analysis are necessary to make the diagnosis. Chest X-ray and computed tomography (CT) are useful for localizing the pleural effusion and planning thoracentesis. Cytology will typically demonstrate triglycerides >110 mg/dL, cholesterol <200 mg/dL, and the presence of chylomicrons (3). Treatment commonly includes low-fat diet modification with or without medications (octreotide, midodrine), followed by surgical correction (thoracic duct and/or leak ligation, pleurodesis, pleurectomy, pleuroperitoneal shunt) if persistent (4). We present the following case of a prolonged chylothorax complicated by breast fistulization refractory to initial therapies. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-157/rc).
Case presentation
We present the case of a 72-year-old female with a 10-year history of recurrent chylothorax with associated lymphedema resistant to initial therapies. During workup for chronic back pain and shortness of breath in 1997, the patient was found to have a pathologic right seventh rib fracture in the location of a cyst. This nonhealing fracture was managed non-operatively for years until she underwent a rib biopsy in 2013 due to concern for fibrous dysplasia, which was complicated by a pneumothorax managed with a chest tube. She developed recurrent chylous pleural effusions that never abated, but she experienced minimal symptoms for the next few years. In 2019, the patient spontaneously experienced increasing right breast and arm swelling, prompting her to visit with multiple cardiothoracic surgeons. Positron-emitting tomography (PET) lymphoscintigraphy documented a lymphatic leak in her right shoulder that communicated with both her right breast and chest. Trialing diuretics, diets, and repeat thoracenteses provided no long-term benefit. With no consensus regarding treatment, she sought input at our institution.
All procedures performed in this study were in accordance with the ethical standards of Mayo Clinic and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
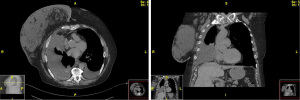
At the time of our initial evaluation, the patient was on a cocktail of medications, including diuretics, inhalers, and opiates. Her lymphedema had mildly improved with compression apparel and a pump, and she denied any leakage from her breast or chest. She experienced dyspnea that prohibited her from walking further than 10 feet without having to stop to catch her breath. Our initial workup included chest CT with intravenous (IV) contrast (Figure 1), ultrasound-guided aspiration of the right breast collection, and magnetic resonance (MR) lymphangiogram of the right breast and bilateral inguinal nodes. Diagnostic thoracentesis revealed an exudative pleural effusion with triglycerides elevated to greater than 2,500 and atypical reactive lymphoid cells negative for malignancy. Microbial analysis was unremarkable. MR lymphangiogram (Figure 2) displayed a diffuse chylous leak into the right pleura spanning T3–11 with abnormal reflux in the T7–T8 intercostal space, resulting in a compensatory lymphatic fistula between the right pleural space and breast with an associated lymphovenous shunt. In light of these findings, our interventional radiology (IR) colleagues opted to proceed with thoracic duct coil and glue embolization around T3–4, as well as IPC placement with right breast aspiration. The patient initially tolerated the procedure well and drained 2.0 L of chyle on the first day.

Over the next few days, she developed diffuse abdominal peritonitis identified as a distal common bile duct leak via magnetic resonance cholangiopancreatography (MRCP), presumably an iatrogenic complication from her embolization. While there were no complications during the procedure, the access point for thoracic duct embolization occurs through the cisterna chyli and bile leaks are known complications. This was managed with endoscopic retrograde cholangiopancreatography (ERCP) and biliary stenting, and she was subsequently discharged shortly thereafter on antibiotics. Six days after discharge, she developed acute bilateral subsegmental pulmonary emboli that was managed with initiation of apixaban. A month out from IPC placement, the patient presented to the emergency department with a three-day history of fever, decreasing output with a change in character, and worsening shortness of breath. Labs revealed leukocytosis with a neutrophilic left shift and reactive thrombocytosis. She was initiated on broad-spectrum antibiotics and administered lytic therapy (dornase and alteplase) through the catheter, resulting in large-volume output. Pleural fluid cultures grew Staphylococcus epidermidis. She was discharged on antibiotics with a plan for surgical intervention the following week.
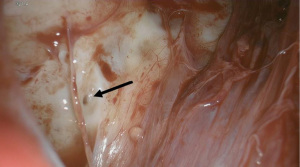
In coordination with our IR colleagues, we proceeded to a hybrid operating room for thoracoscopy and pleurodesis of the lymphorrhea. Lymphazurin blue and water-soluble contrast were injected under sonographic guidance into the right inguinal and right axillary lymph nodes. This was followed by cone beam CT spin, which did not demonstrate any extravasation into the chest. The patient was placed in left lateral decubitus position and we attempted to enter via the eighth intercostal space, but were unable to differentiate lung from chest wall. Another port was placed in the fifth intercostal space and we immediately visualized dense adhesions, necessitating adhesiolysis via carbon dioxide and camera manipulation. We were able to insert our posterior port and the lung was rotated anteriorly to expose the thoracic duct, which had numerous small orifices weeping copious clear fluid suggestive of chyle (Figure 3). These were sealed with DuraSeal (Integra Life Sciences, Princeton, NJ, USA) and talc. Mechanical pleurodesis augmented with poudrage was performed. Three chest tubes were placed within the cavity and an ultrasound-guided Jackson Pratt (JP) drain was placed in the right breast.
Post-operatively, a clear liquid diet with a 1.5 L restriction and low-fat total parenteral nutrition (TPN) were initiated. Non-steroidal anti-inflammatory drugs (NSAIDs) were avoided in pain-control to facilitate pleurodesis and furosemide was given for peripheral edema. Chest tubes were maintained on continuous suction and the output slowly decreased over the coming days. On post-operative day 5, all chest tubes were placed to water-seal. Sinogram of the breast drain shortly thereafter showed filling in the lymphatic malformation of the right breast and axilla for which doxycycline sclerotherapy was performed through the drain. On post-operative day 9, one chest tube was removed, TPN was discontinued, and a low-fat diet was initiated. Another chest tube was removed the following day and the final chest tube was removed on post-operative day 12. Repeat sinogram showed persistent lymphorrhea for which we utilized Sotradecol and doxycycline for sclerotherapy, and she was subsequently discharged home with the breast drain in place afterwards. She followed-up with us for weekly sinograms, until the breast drain was ultimately removed one-month post-discharge (post-operative day 45). She has not experienced any reaccumulation of chyle in the chest or breast to date.
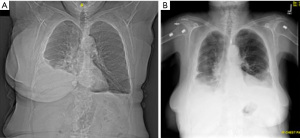
Our management permitted decompression of the breast and prevented reaccumulation of the chylous leaks. The breast tissue returned to a comparable size and appearance as the unaffected side (Figure 4), and her pulmonary function improved drastically. Her medication regimen has decreased to one diuretic and continued opiate use in light of incisional pain attributable to intercostal nerve irritation. Her shortness of breath continues to improve with increasing activity.
Discussion
Herein, we report a case of a recurrent chylothorax that emphasizes the challenges involved in managing lymphatic leaks, particularly in the unique setting of a fistula. Chronic high output chylothoraces carry a 25–50% mortality rate from nutritional, intravascular, and immunologic depletion (5). While this patient was able to compensate with numerous medications and repeat thoracenteses, it was not without cost to her quality of life. Her mobility was severely hindered and she was regularly meeting with palliative care to discuss additional approaches to maximize comfort without addressing the underlying cause.
Optimal management remains unclear in high-output or recalcitrant chylothoraces. Conservative management with nil per os (NPO) and TPN or a low-fat diet has demonstrated efficacy in 81% and 84% of chylothorax patients, respectively (6,7). Procedural intervention is indicated if conservative management fails, output exceeds 500–1,500 mL/day, or it persists past 2 weeks (3,8,9). Minimally invasive lymphatic interventions (lymphangiography, thoracic duct embolization or disruption) have gained increasing popularity considering their reduced morbidity, but are limited to specialized centers and have reduced efficacy when compared with surgery (60.1% versus 64–100%, respectively) (10-12). Such interventions are not without risk as demonstrated in our patient. Considering the access point is through the cisterna chyli, complications commonly include bile leak, guidewire fracture, non-target embolization, and perihepatic hematomas (10). While surgical intervention is largely effective, around 11% of patients require reoperation and identification of the leak is challenging, even with dye (13). Our patient’s case was compounded by holes within the thoracic duct and a fistula, which responded to repeat sclerotherapy. The combination of sclerotherapy (ethanol, tetracyclines, bleomycin, povidone-iodine, Sotradecol) and percutaneous drainage has demonstrated efficacy of 88–100% in lymphorrhea (14,15). The difficulty in managing lymphatic leaks emphasizes the importance of coordinating interdisciplinary care.
At presentation, we required extensive imaging to elucidate the patient’s distorted lymphatic anatomy. This is compounded by the congenital anatomical variance that occurs in 40–60% of patients, further complicating intervention (16). It is difficult to assess the specifics of her previous care without records or pathology results from the outside facility. Piecing together information from the patient and her available medical records, we gleaned a prolonged, undertreated history of chylothorax. Allowing this to fester for years without resolution yielded significant lymphatic expansion into the right breast via fistulization. Though lymphatic fistulas are well-reported, we were unable to find a case of chronic chylothorax forming a shunt to the breast, much less to such a degree (17-20). This distension of the right breast observed pre-operatively was able to return to its normal anatomy at the conclusion of our management.
Upon entry into the chest cavity, we identified extensive adhesions requiring an hour of lysis to visualize the thoracic duct. There were multiple porous channels on the thoracic duct, which increased the difficulty in managing this patient. We could not determine if the patient had a congenital lymphatic malformation superimposed on the traumatic rib biopsy. Alternatively, this could have resulted from an exaggerated immune response to the inciting event, leading to fibrinous obstruction in a mechanism similar to trapped lung syndrome (21). Such occlusive chronicity may have weakened the integrity of the thoracic duct, thereby increasing porosity. Regardless, our intervention was able to resolve the patient’s lymphatic leaks and fistulization without recurrence.
Conclusions
A 72-year-old female experienced significant morbidity after an iatrogenic chylothorax was minimally treated for years. A literature review revealed a dearth of similar reports, highlighting the irregularity in this case. While the optimal management remains debatable, we found success with a multidisciplinary, multimodal surgical approach when resistant to less invasive therapies.
Acknowledgments
We would like to thank Mayo Clinic for providing the resources necessary in this patient’s treatment.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-157/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-157/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-157/coif). J.S.R. receives institutional support via grants from Intuitive and consulting fees from Vergent, Elucent, Noah, and Isola. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of Mayo Clinic and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made. At Mayo Clinic, single subject case studies or a case series with multiple subjects that are prepared and disseminated for educational purposes, are not systematic investigations and, therefore, are not considered research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macfarlane JR, Holman CW. Chylothorax. Am Rev Respir Dis 1972;105:287-91. [PubMed]
- Yang YH, Park SY, Kim DJ. Chyle Leakage after Esophageal Cancer Surgery. Korean J Thorac Cardiovasc Surg 2020;53:191-9. [Crossref] [PubMed]
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med 2010;104:1-8. [Crossref] [PubMed]
- Schild HH, Strassburg CP, Welz A, et al. Treatment options in patients with chylothorax. Dtsch Arztebl Int 2013;110:819-26. [PubMed]
- Agrawal A, Chaddha U, Kaul V, et al. Multidisciplinary Management of Chylothorax. Chest 2022;162:1402-12. [Crossref] [PubMed]
- Takuwa T, Yoshida J, Ono S, et al. Low-fat diet management strategy for chylothorax after pulmonary resection and lymph node dissection for primary lung cancer. J Thorac Cardiovasc Surg 2013;146:571-4. [Crossref] [PubMed]
- Shimizu K, Yoshida J, Nishimura M, et al. Treatment strategy for chylothorax after pulmonary resection and lymph node dissection for lung cancer. J Thorac Cardiovasc Surg 2002;124:499-502. [Crossref] [PubMed]
- Reisenauer JS, Puig CA, Reisenauer CJ, et al. Treatment of Postsurgical Chylothorax. Ann Thorac Surg 2018;105:254-62. [Crossref] [PubMed]
- Marts BC, Naunheim KS, Fiore AC, et al. Conservative versus surgical management of chylothorax. Am J Surg 1992;164:532-4; discussion 534-5. [Crossref] [PubMed]
- Kim PH, Tsauo J, Shin JH. Lymphatic Interventions for Chylothorax: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol 2018;29:194-202.e4. [Crossref] [PubMed]
- Paul S, Altorki NK, Port JL, et al. Surgical management of chylothorax. Thorac Cardiovasc Surg 2009;57:226-8. [Crossref] [PubMed]
- Maldonado F, Cartin-Ceba R, Hawkins FJ, et al. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci 2010;339:314-8. [Crossref] [PubMed]
- Pui MH, Yueh TC. Lymphoscintigraphy in chyluria, chyloperitoneum and chylothorax. J Nucl Med 1998;39:1292-6. [PubMed]
- Lv S, Wang Q, Zhao W, et al. A review of the postoperative lymphatic leakage. Oncotarget 2017;8:69062-75. [Crossref] [PubMed]
- Tasar M, Gulec B, Saglam M, et al. Posttransplant symptomatic lymphocele treatment with percutaneous drainage and ethanol sclerosis: long-term follow-up. Clin Imaging 2005;29:109-16. [Crossref] [PubMed]
- Johnson OW, Chick JF, Chauhan NR, et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol 2016;26:2482-93. [Crossref] [PubMed]
- Singh M, Deo SV, Shukla NK, et al. Chylous fistula after axillary lymph node dissection: incidence, management, and possible cause. Clin Breast Cancer 2011;11:320-4. [Crossref] [PubMed]
- Sakman G, Parsak CK, Demircan O. A rare complication in breast cancer surgery: chylous fistula and its treatment. Acta Chir Belg 2007;107:317-9. [Crossref] [PubMed]
- Donkervoort SC, Roos D, Borgstein PJ. A case of chylous fistula after axillary dissection in breast-conserving treatment for breast cancer. Clin Breast Cancer 2006;7:171-2. [Crossref] [PubMed]
- Tan B, Bloom R. Chylous fistula after sentinel lymph biopsy. J Plast Reconstr Aesthet Surg 2016;69:873-4. [Crossref] [PubMed]
- Upadrista PK, Sabbula BR, Akella J. Trapped Lung. Treasure Island, FL, USA: StatPearls Publishing; 2023.
Cite this article as: Chopko TC, Voppuru SR, Bendel EC, Reisenauer JS. Management of a decade-old recurrent chylothorax with breast fistulization: a case report. AME Case Rep 2024;8:24.


