
Mini laparotomy for candy cane syndrome at the jejunojejunostomy after a second Roux Y Gastric bypass with multiple surgical history: a case report
Highlight box
Key findings
• Even though the candy cane syndrome (CCS) risk is often increased following a Roux-en-Y gastric bypass (RYGB), the risk is not diminished after a successful procedure, but rather might independently correlate with the number of RYGB surgeries and the patient’s past surgical history.
What is known and what is new?
• It is known that CCS is a complication following RYGB.
• CCS can be mistakenly computed tomography-diagnosed as intussusception.
What is the implication, and what should change now?
• The diagnosis of CCS shouldn’t be eliminated after a complication-free RYGB but should be considered each time a patient might need to have another gastric bypass surgery.
• Failure to confirm the diagnosis of intussusception should be a leading point to think about CCS as a differential diagnosis.
Introduction
With the advances in metabolic surgeries and the increasing prevalence of obesity globally, in addition to the proven advantages of preventing health issues, more surgeries are being performed and are advancing worldwide. Candy cane syndrome (CCS) is a rare complication that has been reported in bariatric patients following Roux-en-Y gastric bypass (RYGB). It typically occurs when there is an excessive length of the roux limb proximal to the gastrojejunostomy, which makes it possible for food particles to lodge and reside in the redundant blind limb. The presentation can be vague, with non-specific symptoms including abdominal pain, nausea, and vomiting. Symptoms suggestive of CCS have been reported as early as 3 months to as late as 11 years (1,2). Most remain undiagnosed as the course of the disease is poorly described.
A series of cases regarding CCS in the USA have been reported and successfully managed. All patients presented with non-specific symptoms of small-bowel obstruction (SBO), and tenderness in the epigastric region was consistent on physical examination, the cause of which was negative on pre-operative investigations with esophagogastroduodenoscopy (EGD), computed tomography (CT), or an upper gastrointestinal (GI) study. The cases were managed by resecting the excessive redundancy of the long blind jejunal loop of gastrojejunostomy anastomosis using the Endo GIA Tri-Stapler device. After the procedure, patients were tolerating their diet, completely asymptomatic and pain-free (3).
Although CCS is a well-described entity after laparoscopic-gastric bypass (LGB), often occurring near the gastrojejunal (GJ) junction and causing vague symptoms, it can manifest near the jejunojejunal (JJ) junction, leading to a more severe and acute presentation such as SBO (4).
A research study aimed at evaluating the sensitivity of perioperative diagnostic methods for CCS, in addition to perioperative outcomes and symptom resolution post candy cane resection surgery that was conducted in the United Kingdom. It revised 28 cases of CCS between 2010 and 2017. Among these cases, abdominal pain presenting in (86%), regurgitation/vomiting (43%), suboptimal weight loss (36%), and acid reflux (21%). A total of 73% of patients were found to have complete or partial symptom resolution post CCS surgical revision, while 25% of the cases were found to have further perioperative complications. The utmost success rate was documented in the regurgitation/vomiting subgroup. In terms of CCS size, compared to the pain-free cases, patients who presented with abdominal pain were revealed to have a higher size (4.2 vs. 2 cm). The perioperative tests that examined and delivered a precise diagnosis include barium contrast swallow (63%), upper GI endoscopies (50%), and computer tomographies (29%). CCS can be detected using more than one diagnostic tool and should be used when investigating post-RYGB patients with abdominal symptoms (5).
In this report, we present a patient, who had undergone multiple surgeries in the past, with CCS following a second attempt of gastric bypass surgery, which was mistakenly CT-diagnosed as intussusception. The leading point to diagnose and treat CCS was intussusception. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-62/rc).
Case presentation
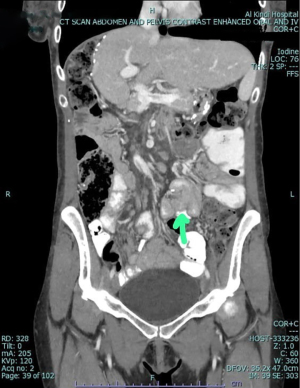
A 34-year-old female, known case of bronchial asthma and trigeminal neuralgia on medications, and without any other medical or genetic conditions. Clinically, the patient presented with nausea, vomiting, food intolerance, acid reflux, appetite loss, and abdominal pain for 1 month, which was associated with intermittent fever and chills, night sweats, shortness of breath, palpations, fatigue, dizziness, and generalized body pain. The patient was admitted for further evaluation and management. The patient had a significant past surgical history: fistulotomy, right hepatectomy without the middle hepatic vein (for donation), ultrasound-guided biopsy of the right sub-phrenic collection, and an excision of a subcutaneous nodule in the abdominal wall. All her past surgeries were successfully completed without any significant subsequent significant complications. On examination, there was tenderness at the epigastric area and the groin with mild abdominal distension. An EGD was done following the second gastric bypass surgery with the impression of a lax gastroesophageal junction, healed GJ ulceration with mild fundal erythema. Then, a CT scan was performed, which raised the possibility of intussusception. Based on CT findings, the surgeon proceeded with a diagnostic laparoscopy, where extensive adhesions were discovered, thus, adhesiolysis was performed. During the adhesiolysis process, a blind-end extra-bowel loop was seen near the JJ anastomosis surrounded by a large number of adhesions, this obligated the surgeon to perform a mini-laparotomy, where a blind-loop of 14 cm was resected (Figure 1), the anastomosis was resolved, and a complete resolution of symptoms have occurred. The specimen was sent to histopathology lab for further evaluation (Figure 2). Table 1 compares pre-operative and post-operative laboratory findings. Ultrasound and CT scans were done pre-operatively (Figures 3-5).
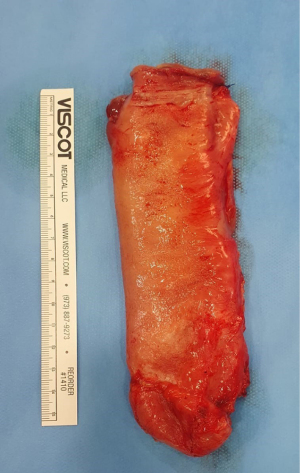

Table 1
| Lab variable | Pre-operative result | Post-operative result |
|---|---|---|
| WBC, 106/µL | 7.21 | 6.59 |
| RBC, 106/µL | 3.8 | 4.1 |
| Hemoglobin, g/dL | 10.9 | 10.6 |
| PLT, 103/µL | 248 | 249 |
| Na, mmol/L | 139 | 140 |
| K, mmol/L | 4.5 | 4.8 |
| Cl, mmol/L | 102 | 102 |
| HCO3, mmol/L | 28 | 28 |
| Urea, mmol/L | 4.5 | 5.6 |
| Creatinine, µmol/L | 44 | 51 |
WBC, white-blood cell; RBC, red-blood cell; PLT, platelets.


All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
As illustrated in the under-reported CCS cases worldwide, patients usually present non-specific GI symptoms (6) along with a typical location; the GJ junction (4). This case is different as obstruction took place at an unusual location; the JJ junction. Additionally, the patient had her first gastric bypass surgery successfully without any subsequent complications for four years. Although the patient had multiple surgeries done before and after her first mini-gastric bypass surgery, CCS was only found incidentally after the second gastric bypass surgery. The EGD was inconclusive and the CT scan preceding the excision of the blind loop reported the impression of an intussusception, based on which the surgeon operated, but rather incidentally discovered the CCS. This case highlights that the risk of CCS is still apparent even after a previous successful gastric bypass surgery. Furthermore, CCS appears to mimic intussusception findings on CT scan reports, which can be misleading to surgeons. Finally, this is the first reported case of CCS in Bahrain, and the second in Gulf-Cooperation Council (GCC) countries, which encourages the need for further studies between possible epidemiological risk factors and CCS in the Middle East region.
Conclusions
Even though CCS risk is often increased following a RYGB, the risk is not diminished after a successful procedure. The risk might as well independently correlate with the number of RYGB surgeries and the past surgical history a patient had undergone.
Acknowledgments
All the authors would like to show their gratitude to Sherif Al Orayedh, Department of Radiology, Al Kindi Hospital, Manama, Kingdom of Bahrain, who has provided us with the CT images and reports needed for this case.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-62/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-62/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-62/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aryaie AH, Fayezizadeh M, Wen Y, et al. "Candy cane syndrome:" an underappre-ciated cause of abdominal pain and nausea after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 2017;13:1501-5. [Crossref] [PubMed]
- Dallal RM, Cottam D. "Candy cane" Roux syndrome--a possible complication after gastric bypass surgery. Surg Obes Relat Dis 2007;3:408-10. [Crossref] [PubMed]
- Khan K, Rodriguez R, Saeed S, et al. A Case series of candy cane limb syndrome after laparoscopic Roux-en-Y gastric bypass. J Surg Case Rep 2018;2018:rjy244. [Crossref] [PubMed]
- Almayouf M, Billa S, Alqahtani A. Candy cane syndrome at jejunojejunostomy causing small bowel obstruction following revisional laparoscopic gastric bypass: A case report and review of literature. Int J Surg Case Rep 2021;86:106360. [Crossref] [PubMed]
- Kamocka A, McGlone ER, Pérez-Pevida B, et al. Candy cane revision after Roux-en-Y gastric bypass. Surg Endosc 2020;34:2076-81. [Crossref] [PubMed]
- Greenberg I, Braun D, Eke C, et al. Successful treatment of "candy cane" syndrome through endoscopic gastrojejunal anastomosis revision. Clin J Gastroenterol 2021;14:1622-5. [Crossref] [PubMed]
Cite this article as: Isa M, Isa A, Alyami A, Alali M, Alalawi M, Salih M, Al-Asiri A, Al-Ghuthayr K. Mini laparotomy for candy cane syndrome at the jejunojejunostomy after a second Roux Y Gastric bypass with multiple surgical history: a case report. AME Case Rep 2024;8:29.


