
A rare tale of an extralobar pulmonary sequestration as a cause of hemoptysis not often contemplated: a case report
Highlight box
Key findings
• Initial atypical symptomatology in our patient with extralobar sequestrations (ELSs) who received resolution of symptoms with non-surgical management—rarely re-ported in literature.
What is known and what is new?
• ELS is the rarest form of bronchopulmonary sequestration (BPS) which often present with severe complications and often require surgical management.
• In our case, our patient presented with a fairly benign episodic hemoptysis as an initial symptom that prompted the resultant diagnosis, and a resolution of symp-toms without the need of surgery. There is also an exciting emerging use of inter-ventional radiology for BPS.
What is the implication, and what should change now?
• It is essential to understand what ELS is, how it typically presents, how it is diagnosed and how its’ management may not always have to be surgical, if its symptoms are mild and could be addressed non-surgically.
Introduction
Bronchopulmonary sequestration (BPS), simply known as pulmonary sequestration, is a rare type of congenital lung malformation of the lower airway, whereby a non-functional mass of lung parenchyma is present but lacks a tracheobronchial connection to the rest of the functioning airway. This mass then receives its arterial blood supply through the systemic circulation (1). Congenital lung malformation account for approximately 10% of all congenital anomalies, while BPS accounts for just 1–3% of all congenital lung malformations (1,2).
This case report describes this rare condition in a 56-year-old healthy female which manifested initially as hemoptysis and aims to raise awareness of this rare form of BPS as a differential for clinicians along with the recommendation for management. This article is presented in accordance with the CARE reporting checklist (3) (available at https://acr.amegroups.com/article/view/10.21037/acr-23-169/rc).
Case presentation
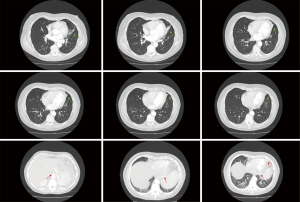
A 56-year-old woman was referred to the respiratory clinic by her general practitioner (GP) after a computed tomography (CT) study with contrast showed an incidental finding of a possible BPS. A second opinion was then sought by the respiratory team from an external thoracic radiologist. The compiled findings from these radiologists for this CT study can be found in Figure 1. This CT study was initially ordered due to infrequent but multiple episodes of hemoptysis, which completely resolved when she was seen in the respiratory clinic. At that point, she was largely asymptomatic except for a degree of exertional dyspnoea which was worked up by a cardiologist, who determined it to be of no cardiac cause. She is otherwise generally healthy and has no constitutional symptoms. She has completely recovered from a recent episode of pneumonia which was managed by her GP with oral antibiotics. Prior to this, the patient did not have a history of any previous or recurrent pneumonias. Her medical history is also notable for hypothyroidism which she is on daily oral thyroxine. She also takes estrogen for her menopausal symptoms. She is not aware of any known birth defects. Collateral history acquired from her mother confirms that she did not have recurrent childhood lung infections and had no known birth defects. She has worked in an office environment as a receptionist for 40 years. Her family history was unremarkable. She was a previous smoker with 15 pack years. On examination, she is of a lean body habitus and does not use any accessory oxygenation devices or puffers. She was afebrile, had a respiratory rate of 12 and her oxygen saturations were 98% at room air. There was no evidence of respiratory distress or chronic hypoxemic states. There was no clubbing or appreciable cervical or axillary lymphadenopathy. There was a dull percussion note with mildly decrease air entry on her left middle to lower zone. The rest of her systems examination was unremarkable.
Bloodwork done showed a mild normocytic anaemia but was confirmed to be at baseline when looking at previous blood results. Her kidney, liver and thyroid functions were unremarkable. Her C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were within normal limits. Autoimmune screening was negative and complement levels were normal. A full lung function test done showed normal respiratory function, with normal spirometry, lung volumes and diffusion capacity.
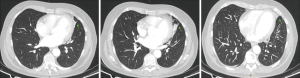
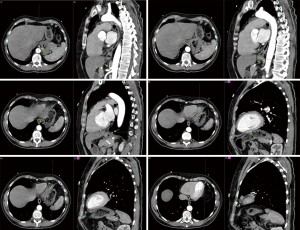
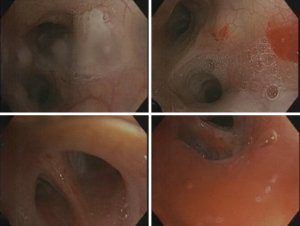
A repeat CT study with contrast once again showed similar findings at the inferior lingula with evidence of an aberrant systemic arterial supply as shown in Figure 2. Based on this CT study, which was compared to the first CT study during reporting, the diagnosis of BPS was then given. The classification of this BPS type being an extralobar sequestration (ELS) was also strongly suggested. In addition, there was also a queried radiological finding which was a possible tracheobronchial connection, and the recommendation for a diagnostic bronchoscopy was given. The findings from the thoracic aortogram done later are shown in Figure 3. A diagnostic bronchoscopy carried out later showed no tracheobronchial connection and an anatomic narrowing of the lumen at the inferior lingula, as shown in Figure 4. Washings done acquired blood-tinged fluid from the lingula segment of the left lung, and the rest of cultures, acid fast bacilli, and cytology was unremarkable. A multidisciplinary meeting involving cardiothoracic surgeons, respiratory physicians and a thoracic radiologist was held and unanimously agreed that as a result of all these above findings, the diagnosis of ELS was given. Option of a biopsy was given during the discussion of management which involved the patient, her family, cardiothoracic surgeons, a cardiologist, and another external respiratory physician. The patient rejected the biopsy after contemplating the risks and the conclusion for management was observation along with activity modification, such as reduction of sympathetic stimulation, e.g., lower intensity exercises, with the hope of decreasing the occurrences of hemoptysis, along with quarterly reviews and yearly CT surveillance. The patient reported a great outcome through conservative management and an absence of further hemoptysis episodes on subsequent reviews.
All procedures performed in this study were in accordance with the ethical standards of the Townsville Hospital and Health Service Human Research Ethics Committee (EX/2023/QTHS/101999) (approved and endorsed on 21 September 2023) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
There are currently four known types of BPS. Intralobar sequestration (ILS), the most common type, is characterized by the sequestered lobe being present within the normal lobe and lacking its own visceral pleura. ELS, is where the sequestered lobe is found outside the normal lobes of the lung and has its’ own visceral pleura. There are other forms of ELS, such as extrathoracic ELS. Congenital pulmonary airway malformation (CPAM), also known as hybrid BPS, is where the malformed lesion is either an ILS or ELS and possessing unique histological features. Lastly, bronchopulmonary foregut malformation is where the sequestered lobe is abnormally connected to the gastrointestinal tract, which happens during the development of the foregut, hence its name (1,4-6). ILS accounts for about 75%, while ELS accounts for 25% of BPS (1,4,7).
The pathogenesis of BPS, though not completely understood, has an embryological basis to many theories (2,8). The concept of sequestrations originating from recurrent infections causing an angiogenic process of aberrant arterial vessels arising from the aorta have also been suggested (9). BPS is often discovered as an incidental finding. The clinical presentation differs in the type of BPS. Half of all adults with ILS are asymptomatic. If symptomatic, ILS commonly presents as recurrent pneumonias due to the absence of the visceral lining. Conversely, ELS do not present with infections, but with rare complications such as respiratory distress, congestive cardiac failure or spontaneous pulmonary haemorrhages (10). In this instance, the cause of the episodic hemoptysis may be due to the high pressure blood flow coming through the abnormal tortuous segments of the arteries stemming from the abdominal aorta during high physiological demand states, e.g., stress or exercising (1,7,11).
Imaging modalities are used to help attain two main objectives. First to exclude other possible causes and second to demonstrate an aberrant arterial supply source (1,5). Typically, ultrasound and/or Doppler ultrasound studies are recommended as the first diagnostic modality for children with a suspected BPS which would appear as an echogenic homogenous mass with evidence of an aberrant blood supply. When chest radiographs are used, it sometimes uncovers odd shaped opacities representing the abnormal aberrant artery, areas of air trapping surrounding the sequestered segment or triangular shaped masses in lung bases (7,12). Resultant complications of lung sequestrations, such as pneumothoraces, pneumonias, atelectasis, and bronchiectasis, may also be found (7,13). On CT studies, much like chest radiographs, the sequestered lung mass may often present as a homogenous or heterogenous mass, a cyst secondary to recurrent infections, lamellar lesion, capsulated lesion with air fluid levels, accompanying emphysema just adjacent to the BPS, cavitary lesion, atelectatic or bronchiectasis segments (2,5,7,12). CT studies are superior in its ability to pinpoint the location and to identify its associated vascular supply, however it may sometimes be dependent on the size of the BPS, hence the use of contrast is usually recommended, as with our case (7,14). A step further to acquire three dimensional reconstructions from the CT studies are often helpful (10). Magnetic resonance imaging (MRI) studies have a place in paediatric populations suspected of BPS due to the absence of ionizing radiation and iodinated contrast material (7). Imaging by itself is often sufficient to make the diagnosis of BPS, but may not always suffice in distinguishing between the four types of BPS. Sometimes, a pathologic assessment once surgically resected is needed (15). Our patient required a diagnostic bronchoscopy; to further ascertain if there was a tracheobronchial connection or fistulous bronchial communication of sorts that may account for her resolved hemoptysis and to determine if it could account for her recent pneumonia episode, whereby her recent pneumonia episode could be the start of recurrent chest infections. The likelihood of this inferior lingula segment of interest in our patient being a congenital development lesion is likely, whereby the high pressure in its tortuous arterial supply may have compromised its function early on in our patient’s life leading to its collapse.
The management option for BPS is surgical resections, that can be done either via thoracostomy or video-assisted thoracoscopic surgery but its typically reserved for symptomatic patients. However, there have been suggestions placed for surgical resection in asymptomatic patients to avoid infection and the rare development of a malignancy (7). A recent tertiary centre retrospective study done showed a complication rate of 28% associated with surgical resections of BPS (4). While another study showed no clear benefit of surgery in asymptomatic patients, as was the case with our patient during our initial and subsequent reviews (16). Often a risk stratification in asymptomatic patients is required prior to deciding a management plan, which involves the size of the BPS along with presence of self or family history of pleuropulmonary blastoma-associated conditions. Observation as a management is sometimes preferred when the lesion is small, non-cystic and consistent with ELS whereby chest radiographs, CT or MRI studies are recommended for ongoing monitoring (2,17). Hence, the decision to surgically resect the sequestered lobe should come down to the analysis of the above, the risks and benefits and most importantly, with patient autonomy. Interestingly, with the rise of interventional radiology, endovascular embolization and coiling have now emerged as possible alternatives, by means of cutting off blood supply to the sequestered lung leading to necrosis and resultant involution (1,16).
Conclusions
In summary, we present a rare cause of infrequent hemoptysis due to an extralobar pulmonary sequestration, found through an incidental finding in an adult female who have been relatively asymptomatic all her life up till this point. A familiarity of aspects of this rare disease should prompt one to consider its’ diagnosis when no other causes are apparent. A combined decision by the clinician and radiologist is usually required to make the diagnosis. The treatment decisions of BPS are multifactorial and should be done coordinatively with multidisciplinary team members, the patient, and their families.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-169/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-169/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-169/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Townsville Hospital and Health Service Human Research Ethics Committee (EX/2023/QTHS/101999) (approved and endorsed on 21 September 2023) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chakraborty RK, Modi P, Sharma S. Pulmonary Sequestration. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Halkic N, Cuénoud PF, Corthésy ME, et al. Pulmonary sequestration: a review of 26 cases. Eur J Cardiothorac Surg 1998;14:127-33. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013;2:38-43. [Crossref] [PubMed]
- Sun X, Xiao Y. Pulmonary sequestration in adult patients: a retrospective study. Eur J Cardiothorac Surg 2015;48:279-82. [Crossref] [PubMed]
- Abbey P, Das CJ, Pangtey GS, et al. Imaging in bronchopulmonary sequestration. J Med Imaging Radiat Oncol 2009;53:22-31. [Crossref] [PubMed]
- Cass DL, Crombleholme TM, Howell LJ, et al. Cystic lung lesions with systemic arterial blood supply: a hybrid of congenital cystic adenomatoid malformation and bronchopulmonary sequestration. J Pediatr Surg 1997;32:986-90. [Crossref] [PubMed]
- Gabelloni M, Faggioni L, Accogli S, et al. Pulmonary sequestration: What the radiologist should know. Clin Imaging 2021;73:61-72. [Crossref] [PubMed]
- Yilmaz A, Bektemur G, Ekinci GH, et al. Extralobar pulmonary sequestration: a case report. Monaldi Arch Chest Dis 2013;79:90-2. [PubMed]
- Lee EY, Boiselle PM, Cleveland RH. Multidetector CT evaluation of congenital lung anomalies. Radiology 2008;247:632-48. [Crossref] [PubMed]
- Chen W, Wagner L, Boyd T, et al. Extralobar pulmonary sequestration presenting with torsion: a case report and review of literature. J Pediatr Surg 2011;46:2025-8. [Crossref] [PubMed]
- Sato Y, Endo S, Saito N, et al. A rare case of extralobar sequestration with hemoptysis. J Thorac Cardiovasc Surg 2004;128:778-9. [Crossref] [PubMed]
- Corbett HJ, Humphrey GM. Pulmonary sequestration. Paediatr Respir Rev 2004;5:59-68. [Crossref] [PubMed]
- Bolca N, Topal U, Bayram S. Bronchopulmonary sequestration: radiologic findings. Eur J Radiol 2004;52:185-91. [Crossref] [PubMed]
- Ikezoe J, Murayama S, Godwin JD, et al. Bronchopulmonary sequestration: CT assessment. Radiology 1990;176:375-9. [Crossref] [PubMed]
- Oermann CM. Bronchopulmonary sequestration. In: UpToDate, Post TW (Ed), Wolters Kluwer. Accessed 9 September 2024.
- Alsumrain M, Ryu JH. Pulmonary sequestration in adults: a retrospective review of resected and unresected cases. BMC Pulm Med 2018;18:97. [Crossref] [PubMed]
- Louie HW, Martin SM, Mulder DG. Pulmonary sequestration: 17-year experience at UCLA. Am Surg 1993;59:801-5. [PubMed]
Cite this article as: Tan C. A rare tale of an extralobar pulmonary sequestration as a cause of hemoptysis not often contemplated: a case report. AME Case Rep 2024;8:35.


