
Multicentric reticulohistiocytosis post-COVID-19: a case report
Highlight box
Key findings
• This case presents the first documented instance of multicentric reticulohistiocytosis (MRH) developing post-coronavirus disease 2019 (COVID-19) infection. MRH can lead to severe joint damage if untreated.
What is known and what is new?
• MRH’s rarity and the COVID-19 link highlight the need for awareness. This case adds novel insights into potential triggers and underscores recognizing atypical presentations.
• The manuscript introduces a unique case that showcases the interaction between viral infections and rare disorders. It stresses considering MRH in COVID-19 patients with skin lesions and joint pain.
What is the implication, and what should change now?
• COVID-19-triggered cytokine cascades might contribute to MRH. Clinicians should proactively assess such patients and conduct biopsies for early diagnosis and intervention.
Introduction
Multicentric reticulohistiocytosis (MRH) is an uncommon systemic granulomatous disorder primarily affecting the skin and joints. The condition displays a higher incidence in females than males, with a ratio of 2:1. The average onset age falls within the range of 40 to 50 years. Saba et al. and Trotta et al. have suggested potential associated conditions such as hyperlipidemia, malignancy, vasculitis, Sjogren’s syndrome, and primary biliary cirrhosis (1,2). Characteristic skin manifestations encompass cutaneous, mucosal, or visceral papulonodular lesions. Joint involvement often takes the form of symmetric polyarthritis, featuring swelling, tenderness, and morning stiffness, frequently impacting proximal interphalangeal (76%), knee (73%), and wrist (64%) joints. In severe instances, radiological findings may indicate destructive patterns (3). Additional prevalent symptoms encompass proximal muscle weakness and general fatigue.
Despite this, the exact pathophysiological mechanism underlying the disease remains unidentified. In this context, we present a case of a woman with no significant medical history, who experienced the onset of MRH following a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. This case report stands as the first documented instance of MRH development subsequent to a SARS-CoV-2 infection. This novel case underscores the limited comprehension of MRH’s pathophysiology and its plausible connection to systemic inflammation. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-144/rc).
Case presentation
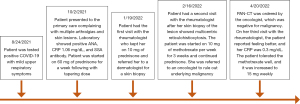
A 38-year-old woman with no medical history presented at the clinic with multiple instances of arthralgia. On August 24, 2021, the patient tested positive for SARS-CoV-2 through a polymerase chain reaction (PCR) test and experienced mild symptoms including rhinorrhea and fatigue. About ten days later, she returned with multiple instances of arthralgia. The patient reported that her shoulders, knees, and hands were the most affected joints. She noted significant tenderness and a decreased range of motion. Although she denied experiencing joint stiffness, the pain persisted throughout the day. Remarkably, she documented the presence of extensive purplish-pink papules, resembling coral beads, on her fingers, nail beds, the volar surface of her fingers, elbows, and within her nostrils (Figure 1). She denied symptoms such as dry eyes, dry mouth, shortness of breath, fever, photosensitivity, Raynaud’s phenomenon, and weight loss.
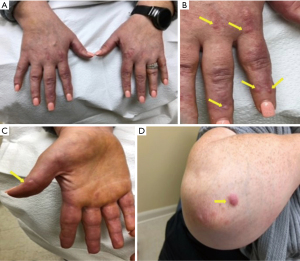
During the examination, bilateral metacarpophalangeal (MCP) joint tenderness and swelling were noted, along with bilateral wrist tenderness and palpable effusion. Despite these findings, her range of motion was intact. Laboratory results showed an antinuclear antibody (ANA) titer of 1:320 with a speckled pattern, a C-reactive protein level of 0.3 mg/dL (normal range: 0.0–0.5 mg/dL), Sjogren’s Syndrome A (SSA) level of 8 (normal: <7), and a negative human immunodeficiency virus (HIV) test. X-ray imaging of her hands did not reveal any signs of erosion (Figure 2).
Due to concerns of potential skin sarcoidosis, a chest X-ray was ordered, which showed no evidence of mediastinal lymphadenopathies. Further laboratory assessments indicated normal levels of vitamin D 25 and 1.25, as well as a negative angiotensin-converting enzyme (ACE) test. The patient was initiated on empiric prednisone at 60 mg daily for four weeks, followed by a taper over the next 4 weeks. Although the steroid treatment notably alleviated the pain, the skin lesions persisted. To clarify the diagnosis, a skin punch biopsy was performed on a nodule on her finger. The biopsy revealed diffuse dermal histiocytic infiltrates indicative of non-Langerhans cell histiocytosis (Figure 3), consistent with a diagnosis reticulohistiocytosis and concerning of MRH.

The patient was started with methotrexate at 15 mg weekly, with a target dose of 20 mg weekly and a subsequent tapering dose of prednisone. The patient was referred to an oncologist who conducted a comprehensive computed tomography scan, revealing no signs of malignancy. Following 3 months of treatment, the patient displayed good tolerance to the methotrexate at 20 mg weekly, and both her pain and skin lesions exhibited improvement (Figure 4).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
MRH is a very rare disease, and the incidence is unknown. There are around 300 cases description in the literature (4). The etiology of MRH remains elusive; however, a plausible hypothesis revolves around the activation of macrophage receptor activator of nuclear factor kappa-B ligand (RANKL) in the skin and joints. Recent findings indicate that the immunomarkers associated with MRH’s pathological cells stem from the monocyte/macrophage lineage. Elevated concentrations of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) within the synovial fluid heighten osteoclastic cell activity through the RANKL signaling pathway. Consequently, this leads to the formation of osteoclast cells and lacunar resorption (5,6).
A comprehensive systematic review by Gracia-Ramos et al. identified over 99 cases of new onset rheumatic diseases following coronavirus disease 2019 (COVID-19). Conditions included reactive arthritis, Kawasaki-like disease, antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis, cutaneous vasculitis, idiopathic inflammatory myopathies, systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA) (7). The phenomenon might be attributed to cross-reactive T-cell recognition and epitope formation. Notably, COVID-19 shares regions similar to 28 human proteins, potentially contributing to autoantibody formation and hyperinflammation, thus triggering neutrophil extracellular trap development.
One in four cases of MRH cases is associated with malignancy such as lung, stomach, breast, cervix, colon, and ovary (8). In our specific case, the patient had a clean medical history and negative results in cancer surveillance tests. The distinctive factor was a positive COVID-19 test. A possible rationale could be linked to an immune system hyperactivation reminiscent of a cytokine storm. According to Sapra et al., COVID-19 patients exhibit prolonged inflammatory reactions that adversely impact bone metabolism (9). Furthermore, COVID-19 might escalate the populations of dendritic cells, macrophages, and adaptive T-cells, ultimately triggering a cascade of immune overactivation and the release of pro-inflammatory cytokines—such as IL-1β, IL-6, IL-17, and TNF-α—via the activation of the RANKL signaling pathway.
In light of the work by Zou et al., four out of 11 patients displayed positive anti-SSA and seven out of 11 had positive ANA levels, suggesting a plausible association with connective tissue disease (CTD) (3).
Clinical presentation
Just as observed in our case, the prevailing discovery concerning skin lesions typically involves brown, purple, reddish, or flesh-colored papulonodular lesions described as coral bead sign (10). These are commonly found on the dorsa of the hands (72.5%, often near the knuckles and periungual region), arms (72.7%), face (54.5%), and auricle (54.5%).
Articular involvement manifests symmetrically and spans a spectrum from mild polyarthralgia to erosive polyarthritis. In certain instances, if untreated, it can even escalate to rapidly destructive arthritis mutilans.
Furthermore, an array of additional symptoms has been documented, encompassing weight loss, weakness, dysphagia, fatigue, fever, myalgia, and muscle atrophy. In a review by Tariq et al., the prevalence of concomitant Sjogren’s syndrome was noted, along with ANA and anti-SSA (Ro) antibodies—similar to our case (11).
The differential diagnosis is extensive. From an arthritis standpoint, conditions such as RA, psoriatic arthritis, Reiter syndrome, and gout should be considered. On the dermatologic front, differential possibilities include xanthomatotic disorders, juvenile xanthogranuloma, eruptive histiocytosis, neurofibromatosis, and sarcoidosis.
Histology finding
The characteristic histological depiction in classical cases involves multinucleated giant cells resembling foreign bodies (12). These cells exhibit a distinctive granulated, ground-glass appearance with eosinophilic cytoplasm. Immunohistochemically, they tend to express CD4, CD5, CD68, HLA-DR, lysozyme, and alpha 1 antitrypsin, while showing negativity for CD20, S100, and factor XIIIa. It is noteworthy that certain instances have reported positive anti-S100 staining, indicating a potential non-dermal origin or involvement of non-Langerhans cells. This divergence in findings across the literature suggests a spectrum of disease rather than a singular MRH entity (13).
In synovial biopsy-based case reports, an observance of nodular infiltrates comprising plump histiocytes with abundant granular eosinophilic cytoplasm has been noted. These nodules also house giant multinucleated cells, marked by positive CD68 immunochemistry and negative S-100 staining (14).
Treatment
Given the rarity of this condition, comprehensive guidelines for its treatment are lacking. Case reports predominantly detail a treatment approach involving steroids in conjunction with disease-modifying antirheumatic drugs (DMARDs), with some cases incorporating bisphosphonates. Bisphosphonates play a role in disrupting the enzymatic functions of intracellular osteoclasts, which could be a pivotal factor in the development of arthritis mutilans. Among these therapies, methotrexate emerged as the most widely employed option, achieving complete symptom resolution in 28% of cases. In instances of severe and refractory cases, cyclophosphamide usage led to resolution in 40% of cases (11). For more recalcitrant diseases, biologic treatments have gained traction. These encompass TNF inhibitors like infliximab, etanercept, and adalimumab. Relatively less commonly used but still considered are tocilizumab and rituximab as alternative treatments.
Conclusions
This case underscores the potential association between COVID-19 and the subsequent development of MRH, highlighting the importance of vigilance in recognizing unique presentations of rare disorders, especially following viral infections. The clinical impact of the case is that MRH is an infrequent yet severe disorder that, when untreated, can manifest as arthritis mutilans, causing irreversible joint damage. Clinicians should be aware of the potential link between COVID-19 and MRH, enabling prompt diagnosis and intervention. The potential implications are that the observed cytokine cascades triggered by COVID-19 may contribute to MRH’s development, emphasizing the intricate interplay between viral infections and autoimmune reactions. Early recognition and biopsy-guided diagnosis could lead to timely treatment, averting long-term consequences.
In summary, our case bridges rare systemic disorders with the post-COVID-19 aftermath. It showcases MRH’s intricate interplay of symptoms, diagnosis, and therapy. Being the first known instance of MRH post-COVID-19, it emphasizes its consideration in similar cases. Successful management offers insights into treating MRH following viral infections. This case contributes to medical understanding, shedding light on MRH’s manifestations and its interaction with viral triggers. In light of these insights, clinicians should remain vigilant in assessing patients who contract COVID-19 and subsequently develop skin lesions and generalized polyarthralgia, advocating for skin lesion biopsies whenever feasible. This proactive approach could lead to early diagnosis and improved patient outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-144/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-144/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-144/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saba R, Kwatra SG, Upadhyay B, et al. Multicentric reticulohistiocytosis presenting with papulonodular skin lesions and arthritis mutilans. Case Rep Rheumatol 2013;2013:201563. [Crossref] [PubMed]
- Trotta F, Castellino G, Lo Monaco A. Multicentric reticulohistiocytosis. Best Pract Res Clin Rheumatol 2004;18:759-72. [Crossref] [PubMed]
- Zou XJ, Qiao L, Li F, et al. Clinical characteristics of multicentric reticulohistiocytosis and distinguished features from rheumatoid arthritis: a single-center experience in China. Orphanet J Rare Dis 2022;17:164. [Crossref] [PubMed]
- Sanchez-Alvarez C, Sandhu AS, Crowson CS, et al. Multicentric reticulohistiocytosis: the Mayo Clinic experience (1980-2017). Rheumatology (Oxford) 2020;59:1898-905. [Crossref] [PubMed]
- Bennàssar A, Mas A, Guilabert A, et al. Multicentric reticulohistiocytosis with elevated cytokine serum levels. J Dermatol 2011;38:905-10. [Crossref] [PubMed]
- Adamopoulos IE, Wordsworth PB, Edwards JR, et al. Osteoclast differentiation and bone resorption in multicentric reticulohistiocytosis. Hum Pathol 2006;37:1176-85. [Crossref] [PubMed]
- Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells 2021;10:3592. [Crossref] [PubMed]
- Shah SP, Shah AM, Prajapati SM, et al. Multicentric reticulohistiocytosis. Indian Dermatol Online J 2011;2:85-7. [Crossref] [PubMed]
- Sapra L, Saini C, Garg B, et al. Long-term implications of COVID-19 on bone health: pathophysiology and therapeutics. Inflamm Res 2022;71:1025-40. [Crossref] [PubMed]
- Sarkar S, Fung MA, Raychaudhuri SP. "Coral bead sign" in Multicentric Reticulohistiocytosis. Int J Dermatol 2020;59:e203-4. [Crossref] [PubMed]
- Tariq S, Hugenberg ST, Hirano-Ali SA, et al. Multicentric reticulohistiocytosis (MRH): case report with review of literature between 1991 and 2014 with in depth analysis of various treatment regimens and outcomes. Springerplus 2016;5:180. [Crossref] [PubMed]
- Bonometti A, Berti E. for Associazione Italiana Ricerca Istiocitosi ONLUS. Reticulohistiocytoses: a revision of the full spectrum. J Eur Acad Dermatol Venereol 2020;34:1684-94. [Crossref] [PubMed]
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum 2000;43:930-8. [Crossref] [PubMed]
- Asano T, Suzutani K, Watanabe A, et al. The utility of FDG-PET/CT imaging in the evaluation of multicentric reticulohistiocytosis: A case report. Medicine (Baltimore) 2018;97:e11449. [Crossref] [PubMed]
Cite this article as: Cho YM, Ross SV, Mahmood R, Bognar MT. Multicentric reticulohistiocytosis post-COVID-19: a case report. AME Case Rep 2024;8:31.



