Spontaneous osteoporotic vertebral refractures after percutaneous vertebroplasty and kyphoplasty in a patient with rheumatoid arthritis: a case report and literature review
Highlight box
Key findings
• We presented a case of spontaneous recurrent adjacent vertebral fractures in a patient of is osteoporotic vertebral compression fractures (OVCFs) with rheumatoid arthritis (RA). When percutaneous kyphoplasty (PKP) or percutaneous vertebroplasty (PVP) is carried out, the comprehensive treatment should involve anti-osteoporosis therapy, it can decrease the incidence of OVCFs and recurrent vertebral compression fractures.
What is known and what is new?
• RA is a chronic systemic autoimmune disease, it is characterized by early manifestations of osteoporosis, the symptoms of osteoporosis are often more severe.
• RA with OVCFs lead to low back pain and spinal deformation. PKP and PVP are widely used in the treatment of RA with OVCFs. But anti-osteoporosis therapy is necessary, because of recurrent vertebral compression fractures.
What is the implication, and what should change now?
• PKP and PVP can relieve pain and improve life quality of the RA patients with OVCFs. The treatment of RA cannot be stopped, in order to prevent successional spontaneous recurrent vertebral compression fractures, antiosteoporosis therapy cannot be neglected.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease, which is characterized by arthritis of multiple peripheral joints, resulting in significant dysfunction and joint pain. One of the main complications of RA is osteoporosis (1), which can cause low back pain and spinal deformation. One of most common complications of osteoporosis is osteoporotic vertebral compression fractures (OVCFs). It has been reported that 40% of osteoporotic patients has OVCFs. Percutaneous kyphoplasty (PKP) and percutaneous vertebroplasty (PVP) are widely used in the clinical treatment of OVCFs because of their minimally invasive nature and effectiveness. But for RA patients with OVCFs, the symptoms of osteoporosis are more severe, they have a greater risk of secondary fractures in adjacent vertebrae after surgery. There is very little information concerning comprehensive treatment of spontaneous recurrent adjacent vertebral fractures in patients of OVCFs with RA, subsequent to PKP and PVP. Here, we report a case of spontaneous OVCFs with RA in which the patient suffered from persistent pain in the low back without any injury, and underwent one PKP and two PVPs with five cement-augmented lumbar vertebrae. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-112/rc).
Case presentation
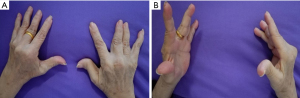
We present the case of a patient who underwent one PKP and two PVPs with five cement-augmented vertebrae, performed in 2014. A 68-year-old woman, who had a 40-year history of RA with knuckle deformity and restricted motion in both hands (Figure 1), which was treated chronically with prednisone all the time, presented with a five-month history of persistent pain in the low back without any injury. The pain treatment with Celecoxib did not improve the condition. All procedures performed in this study were in accordance with the ethical standards of the institutional review board of Yangzhou Clinical Medical College of Nanjing Medical University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
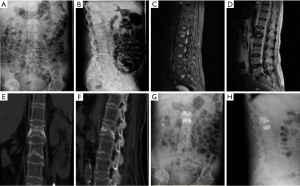
Upon admission, the physical examination revealed widespread tenderness and percussion pain, kyphotic deformity, and restricted motion in the thoracolumbar spine. Laboratory tests showed moderately elevated C-reactive protein (67.44 mg/L), erythrocyte sedimentation rate (27 mm/h), and rheumatoid factor (13.8 IU/mL). Also moderately elevated were the following bone biochemical markers: carboxyterminal telopeptide of type I collagen (CTX; 0.41 ng/mL); N-terminal/mid-molecule (N-MID; 24.04 ng/mL; type 1 procollagen total N-terminal propeptide (TP1NP; 78.76 ng/mL); and total vitamin D (18.77 ng/mL). Plain radiograph, computed tomography (CT), and magnetic resonance imaging (MRI) showed fresh fractures of L1 and L2 (Figure 2). Additionally, T value for bone mineral density (BMD) was −4.1 g/cm2, which suggested osteoporosis; her visual analog scale (VAS) and Oswestry disability index (ODI) scores (2) were 8.0 and 86, respectively. The remainder of her clinical examination was normal. After examination and assessment, minimally invasive surgical techniques were the first choice. We performed PKP at L1 and L2 via a bilateral transpedicular approach (Figure 2), and volumes of bone cement used were 3.5 mL for L1 and 4.0 mL for L2. After the operation, patients received oral Caltrate D and Rocaltrol, and intravenous injection of zoledronic acid (Aclasta) for anti-osteoporosis therapy. The patient showed significant pain relief after the operation and was discharged with improvements in her daily life.
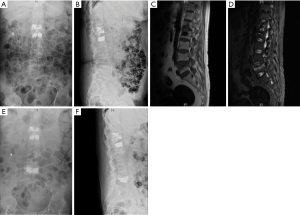
Two months after the PKP, she presented severe pain without injury. MRI showed fresh osteoporotic vertebral compression fractures of L5 (Figure 3). Another round of PVP was performed on the relevant spinal segments, and volumes of bone cement used were 8.0 mL. Postoperatively, we strongly recommended only appropriate activities along with external bracing. The patient showed significant pain relief and improvement in her daily life.
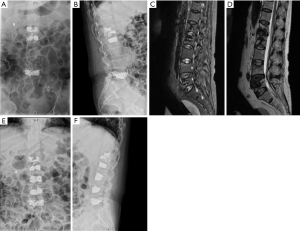
Unfortunately, two months later, she presented with pain at her low back again. MRI revealed a fresh compression fracture of L3 and L4. Again, the third PVP operation was performed at the L3 and L4 (Figure 4). Volumes of bone cement used were 5.0 mL for L3 and 7.0 mL for L4. Since the third PVP operation, the patient was followed up for 36 months with no evidence of lesion recurrence. Patients received three times intravenous injection of zoledronic acid (Aclasta) in all and oral Caltrate D and Rocaltrol for anti-osteoporosis therapy all the time.
Discussion
RA is characterized by early manifestations of osteoporosis (1), because rheumatoid disease leads to bone loss and low BMD (3-5). Some treatments for RA such as corticosteroids are risk factors of osteoporosis (6,7). Physical activity, especially participation in outdoor sports, is significantly reduced due to joint and muscle pain. This affects the synthesis of vitamin D (8). Besides, chronic inflammatory response is the core mechanism of RA pathogenesis, and inflammation itself can also affect the progression of osteoporosis in the general population. A large number of previous studies (9-11) have shown that inflammatory cytokines such as TNF, IL-6, IL-1 and immune cell-derived receptor activator of nuclear factor kappa-B ligand (RANKL) can induce bone loss by directly activating osteoclasts and inhibiting the function of osteoblasts. Anti-citrulline protein antibody (ACPA) is associated with local joint erosion and systemic bone loss in RA. Recent evidence also suggests that high and low ACPA titers are associated with BMD decline, and BMD levels in RA patients with high ACPA titers should be strictly monitored (12).
At present, there is no recommended method to reduce the incidence of osteoporosis and OVCFs in RA patients. For RA patients, clinicians should manage the risk factors related to osteoporosis and OVCFs on the basis of controlling inflammatory response. Non-drug interventions include more physically practice, no smoking and drinking to reduce bone loss (13,14). Also, the patients should get enough calcium and vitamin D everyday (15-17). Clinicians should also administer anti-osteoporosis drugs to patients with decreased BMD. Anti-osteoporosis drugs are mainly anti-bone resorption and osteogenic drugs. Anti-bone resorption drugs aim at reducing bone loss and OVCFs risk. In our case, after the third operation, the patient did not suffer from OVCFs again, therefore, anti-osteoporosis therapy is necessary for RA patients.
The application of PVP or PKP as a therapeutic alternative for the treatment of spontaneous recurrent adjacent-level fractures in patients with RA and OVCFs remains controversial. Fiore (2) recommends posterior percutaneous cement-augmented screws for short fixation in patients with severe osteoporotic vertebral burst fractures. In our case, due to poor physiological function, percutaneous short fixations cannot entirely eliminate the risk of recurrent vertebral fractures. Considering surgical risks, we are inclined to choose PVP and PKP. The objective of PVP or PKP is to enhance the compressive strength and hardness of the vertebral bodies, preventing further collapse, minimizing micromotion at the fracture site, and restoring physiological load transfer (18,19). But in our case, the patient with RA experienced spontaneous multiple OVCFs and underwent one PKP and two PVP operations with five cement-augmented vertebrae from the first to fifth lumbar vertebrae in 5 months. It has been reported that the rate of recurrent vertebral fractures within 1 year after PVP or PKP is as high as 19.59%, and the secondary adjacent vertebral fractures account for 55.17% of the total number of recurrent fractures (19). At the same time, multiple studies have also pointed out that the incidence of secondary fractures of adjacent vertebral bodies is higher in patients after PVP or PKP surgery, which usually occurs within one month after PVP or PKP surgery (20-22). But the adjacent vertebral fractures after PVP or PKP are a natural progression of osteoporosis, which has nothing to do with PVP or PKP (23). The risk of secondary adjacent vertebral fractures can be reduced by 40% after anti-osteoporosis treatment for OVCFs (24). Therefore, standard anti-osteoporosis treatment is very important to RA patients with OVCFs treated with PVP or PKP. It can effectively delay the natural process of osteoporosis, reduce bone loss, improve bone density, and thus facilitate functional reconstruction of the spine and reduce the incidence of adjacent vertebral fractures.
However, the risk of secondary fracture of adjacent vertebrae after PVP or PKP is great .The adjacent vertebral body stress distribution can change, and be strikingly different from the fractured vertebral body in hardness (24). What is more, the stress, relatively concentrated, will transfer to the adjacent vertebral bodies due to trabecular thinning, increasing bone fragility, and a decrease in the number of trabecular elements and trabecular bone volume. This results in a greater incidence of adjacent vertebral fractures (25). Recent data showed that the incidence of new vertebral compression fractures after PVP ranged from 10% to 52% in the following 3 months (18,19,26,27). Other studies have shown that recurrent vertebral compression fractures after PVP are a natural course of osteoporosis, with an incidence that is characteristic of osteoporotic patients (26,28-30). Cement leakage is the most commonly complication in PVP (31). Cement leakage from the broken endplate and into the vertebral disc with no obvious symptom does not need special intervention. However, the increase of stiffness after vertebral cement augmentation can cause a concentration of stress, especially in adjacent intervertebral discs (25,28,29,32). Aggravated intervertebral disc degeneration after vertebral augmentation may increase the recurrence of fresh fractures in adjacent vertebrae; the change in stiffness and distribution of stresses through the augmented vertebrae may contribute to intervertebral disc degeneration (32). A large volume of injected cement restores the strength of the involved vertebrae, but can lead to an induced fracture in adjacent vertebrae (18,28,30,33). Bone cement augmentation increases subsidence in the adjacent vertebral bodies, indicating a great amount of bone cement is related to recurrent adjacent level fractures (19,28,30,33). It has been reported that recurrent adjacent fractures after PVP operation may be related to the recovery of anterior vertebral body height. Excessive height restoration of cemented vertebrae is a reported risk factor for recollapse (18,32). Currently, polymethyl methacrylate used for vertebral cement augmentation has been associated with a change in load-bearing of the intervertebral disc adjacent to the treated vertebrae (18,28). Uneven load transport to the adjacent vertebral endplate results in fresh vertebral fractures (34).
Conclusions
In conclusion, RA is a chronic systemic autoimmune disease, it is characterized by early manifestations of osteoporosis and OVCFs are one of most common complications of osteoporosis. When we treat RA with OVCFs, the comprehensive treatment is very important, PVP and PKP can relieve pain and improve life quality, but anti-osteoporosis therapy is also necessary. Many factors contribute to recurrent vertebral compression fractures in RA patients with OVCFs, such as RA, drugs, osteoporosis, volume of injected cement, height restoration, distribution of stresses, uneven load, and so on. Anti-osteoporosis therapy can decrease the incidence of OVCFs and recurrent vertebral compression fractures.
Acknowledgments
We thank the patient and her family for their active cooperation in follow-up.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-112/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-112/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-112/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roussy JP, Bessette L, Bernatsky S, et al. Rates of non-vertebral osteoporotic fractures in rheumatoid arthritis and postfracture osteoporosis care in a period of evolving clinical practice guidelines. Calcif Tissue Int 2014;95:8-18. [Crossref] [PubMed]
- Fiore G, Tariciotti L, Borsa S, et al. Percutaneous Cement-Augmented Screws Short Fixation for the Treatment of Severe Osteoporotic Vertebral Burst Fractures. World Neurosurg 2022;163:e522-31. [Crossref] [PubMed]
- Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis 2002;61:ii84-6. [Crossref] [PubMed]
- Roux C. Osteoporosis in inflammatory joint diseases. Osteoporos Int 2011;22:421-33. [Crossref] [PubMed]
- Shankar S, Handa R, Aneja R, et al. Bone mineral density in Indian women with rheumatoid arthritis. Rheumatol Int 2009;29:377-81. [Crossref] [PubMed]
- Hardy RS, Raza K, Cooper MS. Glucocorticoid metabolism in rheumatoid arthritis. Ann N Y Acad Sci 2014;1318:18-26. [Crossref] [PubMed]
- Vis M, Güler-Yüksel M, Lems WF. Can bone loss in rheumatoid arthritis be prevented? Osteoporos Int 2013;24:2541-53. [Crossref] [PubMed]
- Hoes JN, Bultink IE, Lems WF. Management of osteoporosis in rheumatoid arthritis patients. Expert Opin Pharmacother 2015;16:559-71. [Crossref] [PubMed]
- Anthamatten A, Parish A. Clinical Update on Osteoporosis. J Midwifery Womens Health 2019;64:265-75. [Crossref] [PubMed]
- Lin YY, Jean YH, Lee HP, et al. Excavatolide B Attenuates Rheumatoid Arthritis through the Inhibition of Osteoclastogenesis. Mar Drugs 2017;15:9. [Crossref] [PubMed]
- Yedavally-Yellayi S, Ho AM, Patalinghug EM. Update on Osteoporosis. Prim Care 2019;46:175-90. [Crossref] [PubMed]
- Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 2012;8:656-64. [Crossref] [PubMed]
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract 2016;22:1-42. [Crossref] [PubMed]
- Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017;12:43. [Crossref] [PubMed]
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol 2017;69:1521-37. [Crossref] [PubMed]
- Harrison SR, Li D, Jeffery LE, et al. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif Tissue Int 2020;106:58-75. [Crossref] [PubMed]
- Song GG, Bae SC, Lee YH. Association between vitamin D intake and the risk of rheumatoid arthritis: a meta-analysis. Clin Rheumatol 2012;31:1733-9. [Crossref] [PubMed]
- Ma X, Xing D, Ma J, et al. Risk factors for new vertebral compression fractures after percutaneous vertebroplasty: qualitative evidence synthesized from a systematic review. Spine (Phila Pa 1976) 2013;38:E713-22. [Crossref] [PubMed]
- Yi X, Lu H, Tian F, et al. Recompression in new levels after percutaneous vertebroplasty and kyphoplasty compared with conservative treatment. Arch Orthop Trauma Surg 2014;134:21-30. [Crossref] [PubMed]
- Aquarius R, van der Zijden AM, Homminga J, et al. Does bone cement in percutaneous vertebroplasty act as a stress riser? Spine (Phila Pa 1976) 2013;38:2092-7. [Crossref] [PubMed]
- Liu WG, He SC, Deng G, et al. Risk factors for new vertebral fractures after percutaneous vertebroplasty in patients with osteoporosis: a prospective study. J Vasc Interv Radiol 2012;23:1143-9. [Crossref] [PubMed]
- Schulte TL, Keiler A, Riechelmann F, et al. Biomechanical comparison of vertebral augmentation with silicone and PMMA cement and two filling grades. Eur Spine J 2013;22:2695-701. [Crossref] [PubMed]
- Sun G, Tang H, Li M, et al. Analysis of risk factors of subsequent fractures after vertebroplasty. Eur Spine J 2014;23:1339-45. [Crossref] [PubMed]
- Bawa HS, Weick J, Dirschl DR. Anti-Osteoporotic Therapy After Fragility Fracture Lowers Rate of Subsequent Fracture: Analysis of a Large Population Sample. J Bone Joint Surg Am 2015;97:1555-62. [Crossref] [PubMed]
- Trout AT, Kallmes DF. Does vertebroplasty cause incident vertebral fractures? A review of available data. AJNR Am J Neuroradiol 2006;27:1397-403. [PubMed]
- Mazzantini M, Carpeggiani P, d'Ascanio A, et al. Long-term prospective study of osteoporotic patients treated with percutaneous vertebroplasty after fragility fractures. Osteoporos Int 2011;22:1599-607. [Crossref] [PubMed]
- Ren HL, Jiang JM, Chen JT, et al. Risk factors of new symptomatic vertebral compression fractures in osteoporotic patients undergone percutaneous vertebroplasty. Eur Spine J 2015;24:750-8. [Crossref] [PubMed]
- Chen LH, Hsieh MK, Liao JC, et al. Repeated percutaneous vertebroplasty for refracture of cemented vertebrae. Arch Orthop Trauma Surg 2011;131:927-33. [Crossref] [PubMed]
- Su CH, Tu PH, Yang TC, et al. Comparison of the therapeutic effect of teriparatide with that of combined vertebroplasty with antiresorptive agents for the treatment of new-onset adjacent vertebral compression fracture after percutaneous vertebroplasty. J Spinal Disord Tech 2013;26:200-6. [Crossref] [PubMed]
- Takahara K, Kamimura M, Moriya H, et al. Risk factors of adjacent vertebral collapse after percutaneous vertebroplasty for osteoporotic vertebral fracture in postmenopausal women. BMC Musculoskelet Disord 2016;17:12. [Crossref] [PubMed]
- Hulme PA, Krebs J, Ferguson SJ, et al. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine (Phila Pa 1976) 2006;31:1983-2001. [Crossref] [PubMed]
- Lazáry A, Speer G, Varga PP, et al. Effect of vertebroplasty filler materials on viability and gene expression of human nucleus pulposus cells. J Orthop Res 2008;26:601-7. [Crossref] [PubMed]
- Lee DG, Park CK, Park CJ, et al. Analysis of Risk Factors Causing New Symptomatic Vertebral Compression Fractures After Percutaneous Vertebroplasty for Painful Osteoporotic Vertebral Compression Fractures: A 4-year Follow-up. J Spinal Disord Tech 2015;28:E578-83. [Crossref] [PubMed]
- Boger A, Heini P, Windolf M, et al. Adjacent vertebral failure after vertebroplasty: a biomechanical study of low-modulus PMMA cement. Eur Spine J 2007;16:2118-25. [Crossref] [PubMed]
Cite this article as: Wen D, Guo D. Spontaneous osteoporotic vertebral refractures after percutaneous vertebroplasty and kyphoplasty in a patient with rheumatoid arthritis: a case report and literature review. AME Case Rep 2024;8:52.