
Diagnosis of Inverted Meckel’s diverticulum by double-balloon enteroscopy: a case report
Highlight box
Key findings
• Double-balloon enteroscopy (DBE) has high clinical application value in the diagnosis of Meckel’s diverticulum (MD).
What is known and what is new?
• MD is the most common congenital digestive tract malformation, and complications such as diverticulitis, gastrointestinal bleeding, and intestinal obstruction may develop. Preoperative diagnosis of MD is challenging due to the nonspecific nature of symptoms.
• Technetium-99 nuclide scanning is usually used for diagnosis. Here, we report that DBE increases accuracy of diagnosis of MD, with several added benefits.
What is the implication, and what should change now?
• Incorporating the use of DBE in the event of a negative technetium 99 nuclide scanning imaging may help in the diagnosis of MD in cases of unexplained lower gastrointestinal bleeding in children.
Introduction
Meckel’s diverticulum (MD) is the most common congenital digestive tract malformation, which is caused by the incomplete degeneration of the yolk duct during fetal development. MD often occurs in the contralateral edge of the mesangium at the end of the ileum. It is a true intestinal diverticulum, which often contains ectopic tissues, the most common being gastric tissue, followed by pancreatic tissues. In a few rare cases, duodenum and colon tissue can also be found (1). The incidence of MD in the general population ranges from 0.3–2.9%, with up to 85% of patients with no clinical symptoms (1,2). Of the 15% who develop complications and symptoms, the symptoms can include nausea, vomiting, intestinal bleeding and abdominal pains, which are nonspecific symptoms shared with other common ailments such as appendicitis, enteritis, peptic ulcers and colic. Serious complications such as diverticulitis, gastrointestinal bleeding and intussusception can develop from MD (3).
The serious and life-threatening complications from MD highlights the importance of accurate diagnosis. However, due to the rarity of the condition, coupled with nonspecific symptoms, preoperative misdiagnoses is possible. Current preoperative diagnosis involves using Technetium-99m pertechnetate scanning. However, such scanning may give false negative results for a true MD with the presence of ectopic pancreatic tissue. Here, we suggest incorporating double-balloon enteroscopy, which is a reliable tool for small bowel imaging, in the diagnosis of MD in cases with negative Technetium-99m pertechnetate scanning results. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-102/rc).
Case presentation
General information
A 12-year-old male child presented with recurrent abdominal pain, and incidence of black stools for more than half a year, which had been recurrent for 2 days. The child had been evaluated and treated for similar complaints in the past. More than half a year ago, he presented to other hospitals with no obvious cause of abdominal pain and black stools, and was hospitalized for acute secondary peritonitis, intestinal obstruction and intussusception. He was discharged after air enema reduction. More than 2 months ago, the child developed pale face and pale lips, tarred defecation, with vomiting and belching, and was hospitalized at our hospital. He received blood transfusion and hemostasis and was discharged with improvement. For the latest presentation at our hospital, there was no obvious cause for the abdominal pain which developed 2 days ago. The child had non-radiating paroxysmal periumbilical pain, with light abdominal tenderness around the umbilicus, with no rebound pain. The pain was unrelated with food consumption, which was slightly relieved by bending over. The pain was accompanied by vomiting, all of which were stomach contents without coffee-like substance and ejection vomiting. There was also abdominal distension, diarrhea, acid regurgitation, belching, black stools, and thirst. The child had no fever, cough, sputum, nasal congestion, runny nose and shortness of breath. An abdominal ultrasound was performed which revealed a heterogeneous mass in the right lower abdomen and intussusception was considered. The patient also had slightly enlarged mesenteric lymph nodes. Symptomatic treatments such as anti-infection, acid suppression and enema were given, and the abdominal pain was relieved. Since the onset of the disease, the child had clear consciousness, good sleep, poor appetite, normal bowel and urine, poor physical strength, and no significant change in weight. The child had no significant previous medical history other than allergic rhinitis. There was no significant family medical history. Physical examination showed normal development, medium nutrition, clear consciousness and mental state. The skin mucosa of the whole body has no pallor, no yellow stain and no bleeding points. Superficial lymph nodes were not enlarged. Heart rate 80 beats per minute. Abdominal tenderness, light tenderness around the umbilicus, no rebound pain, no mass, liver and spleen. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The study was reviewed and approved by Ethics Committee of Jiangmen Clinical College of Guangdong Medical University/Jiangmen Central Hospital (approval number: [2023]4). Written informed consent was obtained from the patient and his parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Auxiliary inspection
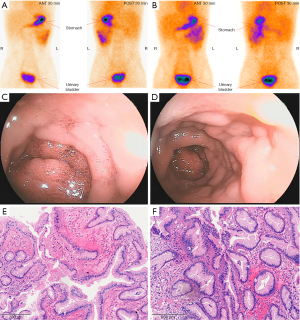
Emergency gastroscopy suggested superficial gastritis. Enhanced computed tomography (CT) showed uniform thickening of distal ileum intestinal wall, with a hint of inflammation changes. Preliminary colonoscopy showed no abnormal colonic mucosa. Examination of bone marrow cytology showed abnormalities. Small intestine magnetic resonance enterography (MRE) showed uniform thickening of the wall of the lower ileum and ileocolon intussusception was observed. The results of the first 99TcmO4-single-photon emission computed tomography/CT (SPECT/CT) imaging conducted were negative (Figure 1A), which was consistent with the second 99TcmO4-SPECT/CT imaging during the recent hospitalisation (Figure 1B). The first and second groups of small intestine imaging agents were diffused and concentrated in the left middle and upper abdomen (Figure 1A,1B), and the corresponding intestinal lumen was slightly dilated and hydrostatic. Double-balloon enteroscopy (DBE) revealed that there was a mass in the upper ileum and multiple mucosal eminence in the upper ileum and multiple mucosal eminence at the terminal ileum (Figure 1C,1D). Pathological examination of the ileal mass showed that the surface of the mass was covered by mucous columnar epithelium (Figure 1E,1F). Significantly, the diffuse concentration distribution of small intestine imaging agents in the first and second groups of the left mid and upper abdomen before surgery overlayed with the sites with mass found in double-balloon enteroscopy.
Treatment
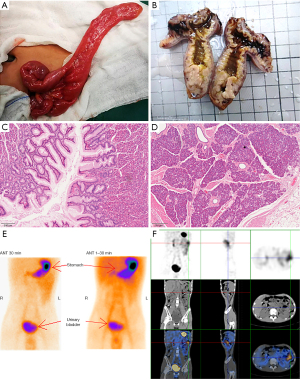
The patient underwent colonoscopy combined with laparoscopic diverticula resection under tracheal anesthesia. Under general tracheal anesthesia, the child was placed in the left decubitus position, and colonoscopy was performed through the anus, sigmoid colon, descending colon, transverse colon, descending colon, to the ileocecal part. No mass, ulcer, or bleeding was found in the colon through the scope. The patient was restored to supine position, and the operative field was routinely disinfected. Subumbilical puncture was performed to establish pneumoperitoneum successfully, and a 5-mm disposable cannula and laparoscope were successfully entered. A 5-mm cannula was inserted into left lower abdomen and left upper abdomen respectively. No abnormality was found in ileocecal part, and the small intestine was explored proximal. A tubular structure, about 6 cm long, was found at the opposite edge of the mesentery about 90 cm from ileocecal part, which was communicated with the intestinal tube, and diverticula was considered. A transverse incision, about 5 cm long, was made at the surface projection of the right lower abdominal diverticulum, which was entered into the abdomen layer by layer. The diseased small intestine segment, diverticulum and part of the small intestine were removed (Figure 2A,2B), the mesenteric vessels of the diseased segment were ligated, and the broken ends were anastomosed to check the anastomosis. No abnormalities were found in other abdominal organs and no active bleeding was observed. After successful resection and surgery were completed, the surgical specimens were sent for pathological examination. Postoperative diagnosis showed that it was small intestine diverticulum, ileocolon intussusception and bile reflux gastritis. Postoperative management measures include monitoring, gastrointestinal decompression, anti-infection, and symptomatic support treatment.
Diagnosis and pathological findings
The final diagnosis was small intestine diverticulum, intussusception, and bile reflux gastritis. Pathological examination of the removed diverticulum showed no significant abnormalities, and the mucosal glands present were fundus gastric glands. Chronic inflammatory changes were found in the mucosa of the surrounding small intestine, and pancreatic lobules, duct structure, acine and scattered islets were found in the musculi proper (Figure 2C,2D). The diverticula is hence concluded to contain both gastric mucosa and pancreatic tissue.
Follow-up and outcome of treatment
After the operation, the symptomatic support was strengthened, and the abdominal pain was improved. The patient recovered well.99TcmO4-SPECT/CT imaging reexamination was performed on June 28, 2022, and both the concentration degree of the imaging agent and effusion of intestinal dilation were less than before (Figure 2E,2F).
A brief timeline with the course of events since the first presentation of abdominal pain in the patient, until diagnosis and treatment is illustrated in Figure 3.

Discussion
The prevalence of MD in the general population ranges from 0.3–2.9%, but as many as 85% of patients were asymptomatic (1,2). MD may develop symptomatic complications such as intestinal obstruction, diverticulitis, intestinal bleeding, and perforation. Only one-third of patients can be definitively diagnosed with MD before surgery, and about one-third of MD is discovered by chance during surgery performed for other reasons (4,5). At present, these are the following diagnostic methods for MD, and there are pros and cons for each method:
99TcmO4-nuclide scanning imaging
99TcmO4 has a special affinity for gastric mucosal wall cells and can be absorbed by gastric mucosa, causing local radioactive concentration areas, so 99TcmO4-nuclide scanning imaging has become an important means for MD diagnosis. 99TcmO4-nuclide scanning imaging for MD has a sensitivity of 80–90%, specificity of about 95% and an accuracy of 90% (2,6). 99TcmO4-nuclide scanning imaging is highly sensitive (75%) in children with fresh, painless small intestinal bleeding, especially in cases of abdominal symptoms accompanied by gastrointestinal bleeding, but is of limited use in diagnosing MD with other abdominal symptoms. However, there are symptoms of gastric mucosa complicated with bleeding in MD, and false negative scanning may also occur (4,5). Spottswood et al. (7) have suggested that the application of this method in the detection of ectopic gastric mucosa with small tissue (<1.8 cm2), vagal diverticulum and rapid gastrointestinal bleeding often results in false negative results. Other scholars (8) have also confirmed that 99TcmO4-is easily dissipated by washing during massive bleeding in the lower digestive tract, resulting in false negatives. Dolezal and Vizda (8) found that when anemia caused by lower digestive tract bleeding reaches a certain level (Hb <110 g/L), the diagnostic sensitivity of 99TcmO4-nuclide scanning imaging in patients with ectopic gastric mucosal imaging MD is lowered to 60%. In our case, 99TcmO4-nuclide scanning imagings were negative both before and after surgery, but the first and second groups of small intestine imaging agents were diffused and concentrated in the left middle and upper abdomen before surgery, and the corresponding intestinal lumen was slightly dilated and hydrostatic. After operation, the concentration degree of imaging agent and the fluid accumulation of intestinal dilation were reduced. As seen in Figures 1,2, the diffuse concentration distribution of small intestine imaging agents in the first and second groups of the left mid and upper abdomen before surgery overlayed with the sites with mass found in double-balloon enteroscopy. Therefore, 99TcmO4-nuclide scanning imaging indicated the distribution of imaging agents in unusual locations, but did not meet the diagnostic conditions for MD. It is worth exploring whether MD should be suspected, and other relevant examinations should be further performed.
Capsule endoscopy
Capsule endoscopy for the diagnosis of MD has been reported in a multi-center I-CARE study in Europe, which suggested that the diagnostic positive rate of capsule endoscopy was 71% (9). However, capsule endoscopy has the risk of retention of diverticulum and the detection rate is limited compared to 99TcmO4-nuclide scanning imaging, so it is not the preferred test for the diagnosis of MD (10).
Imaging examination
Imaging examinations such as ultrasound, X-ray, angiography, CT scan, and MRE can diagnose MD, but with low sensitivity and specificity. For MD complicated with intestinal obstruction, intussusception, small intestine torsion and other complications, imaging examination will be helpful and can lead to early surgical interventions (1). Imaging examination is important for the diagnosis of Inverted Meckel’s diverticulum (IMD), and abdominal ultrasound and CT are the main diagnostic tools. Ultrasound is the first choice for pediatric IMD patients due to its non-invasiveness and non-ionizing radiation. MRE is rarely used to diagnose IMD in children (11).
DBE
DBE can provide visual images of the diverticular internal mouth, which has been proven to be a safe method for the diagnosis of pediatric MD, providing valuable guidance for surgical interventions and greatly improving the safety and effectiveness of surgery (12). The overall diagnosis rate of MD diagnosed by DBE before surgery was 86%, which was significantly higher than that of capsule endoscopy. Compared with radionuclide scanning imaging, its accuracy rate is also higher for MD with suspected symptoms (13). A single-center retrospective study in Taiwan (14) included MD cases diagnosed by DBE and found that the diagnostic rates of other methods besides DBE were 50% for capsule endoscopy, 11% for Meckel’s scan, 16.7% for CT and 33.3% for angiography. It can be seen that DBE has certain advantages in the diagnosis rate of MD compared with other examinations, is a more powerful tool than other routine examinations, and can be used as an important supplement for the examination of digestive tract bleeding of difficult to diagnose causes. DBE can directly observe the diverticulum and comprehensively examine the small intestine, and the bleeding focus can be treated under the microscope. When MD is combined with other lesions, it can also be diagnosed. Endoscopic markers can be used to locate the lesion for surgery. Park et al. (15) have shown that it is difficult to distinguish obvious ectopic tissue in surgical operations (62% of the ectopic tissue of diverticulum is nonpalpable), and there is no relevant research on whether there are differences in the incidence of postoperative complications between resection of diverticulum alone and resection of the diverticulum with the connecting part of the small intestine. Park et al. (15) suggested that the ectopic tissue should be located at the base of the diverticulum. The excision area should be outside the ectopic tissue area. Therefore, preoperative DBE examination and multi-point biopsy of the lesion site and the surrounding histopathology, the detection of ectopic tissue and the determination of its scope can provide key evidence for the diagnosis of MD and the scope of intraoperative resection (12,15). In this child, a mass was found in the ileum during DBE examination, and pathology revealed gastric mucosa and pancreatic tissue, and ectopic gastric mucosa was found in biopsies of the tumor and surrounding intestinal mucosal tissue, suggesting that the lesion site was not limited to the diverticulum part but should exceed the base of the diverticulum, which provided important insights for determining the scope of surgical resection. The diagnostic value of DBE for MD is clear, but the judgment of distance during operation is subjective to some extent, and the operator should pay attention to the judgment to provide positioning help for the subsequent operation. In this case, in the DBE examination, a mass was found in the upper segment of the ileum, but during the operation, the diverticulum was found at the distal end of the ileum, 90 cm away from the ileocecal valve, which was significantly different from the location of the DBE. The reason may be that the normal segment of the long diverticulum inverted and pulled into the intestinal cavity and drifted to the proximal part of the small intestine, resulting in the illusion that the diverticulum was located in the upper segment of the ileum.
During the first hospitalization, while the enhanced CT suggested uniform thickening of the intestinal wall in the distal ileum, we considered that the bleeding focus might be located in the distal ileum, although the possibility of diverticulum, local inflammation and small bowel lymphoma were not excluded, due to the negative nuclide SPECT/CT imaging, we ignored the diverticulum, and the conventional blood results showed decreased leukocytes and hemoglobin. Therefore, we looked at the bone marrow cytology, which indicated granulocytopenia (we considered secondary to infection, caused by repeated diverticulitis, inverted small bowel diverticulum, and diverticulitis. Repeated postoperative review of the child’s blood routine without granulocytopenia also confirmed our contention). We suggested DBE or laparotomy, but the child was discharged due to the good treatment response during the hospitalization, with no abdominal pain and bloody stools symptoms, and the family members refused further examination. MRE of the small intestine during the second hospitalization suggested uniform thickening of the unsegmented ileum wall and ileo-colonic intussusception, indicating possible inflammation or tumor. The second 99TcmO4-SPECT/CT imaging result remained negative and still did not support MD. We suggested DBE or surgical exploration to determine the nature of the lesion. After consultation, the medical staff and the family members decided to choose double-balloon enteroscopy, which is a less invasive procedure. The results indicated that the mass of the upper ileum was found, and the pathological return was gastric mucosal tissue. At this time, we considered the MD with ectopic gastric mucosa. The medical staff and the family members explained the condition, and the family members agreed to explore and remove the lesion. MD is mostly located at the opposite edge of the mesentery 20–100 cm from the ileocecal valve, with a large range of favorable sites. There was significant difficulty in locating the lesion in this case. 99TcmO4-SPECT/CT imaging failed to indicate the location of the diverticulum, and although the DBE suggested that the lesion was located in the upper ileal segment, the lesion localization was greatly influenced by the examiner’s manipulation experience, and children have relatively high intestinal activity and poor intestinal fixation, which makes locating the lesion challenging. Moreover, the DBE suggested that the lesion was an intestinal lumen mass with unclear boundary and irregular morphology, without a typical diverticulum opening. Therefore, based on the purpose of surgical exploration, we first chose laparoscopic combined colonoscopy to avoid a large incision to explore the abdominal cavity, and to avoid missing colonic lesions, when the diverticulum is found after open diverticula and intestinal anastomosis.
Park et al. (15) suggested that 75% of MD patients are male, 75% have symptoms when they are above 10 years old, and 75% of MD with bleeding symptoms contain ectopic gastric mucosa. Risk factors for diverticula complications include age of less than 50 years, male, diverticula length of more than 2 cm, and the diverticula containing ectopic or abnormal tissues. Therefore, clinicians who are presented with male children who have recurrent intestinal obstruction, gastrointestinal bleeding, recurrent abdominal pain and other related symptoms should be vigilant against MD. In this case, in addition to gastrointestinal bleeding and intestinal obstruction, the child also had repeated intussusception, which highly points towards MD. Many attempts were made to have a clear diagnosis, which includes small intestine MRE, abdominal enhanced CT, gastroenteroscopy, etc., but all failed to make a clear diagnosis of MD. The diagnostic methods of MD, including ultrasound, MRE, CT, technetium-99 nuclide scanning imaging, have certain limitations: ultrasound, MRE and CT have clear effects on the diagnosis of MD complicated with intestinal obstruction, but cannot determine whether the cause of intestinal obstruction is MD. Due to their limited detection range, colonoscopy and gastroscopy cannot reach the small intestine, which is the most common site of MD. Pathological diagnosis is the key to the judgment of ectopic tissue, and the complications and severity caused by different ectopic tissue and diverticulum size and scope are related. At present, DBE is not widely used in the diagnosis of MD. Compared with the above examinations, it has a higher accuracy and can reach the small intestine. For patients with bleeding in MD, DBE can directly detect and observe the shape, size and lesion of the diverticulum, and can also take pathological examination and hemostasis under the microscope, and can mark the lesion site and scope for surgery. It has high clinical application value.
Park et al. (15) found that ectopic tissue is found in about 29% of MD, and most of the ectopic tissue in MD is gastric mucosa, accounting for about 60–85% of cases, while pancreatic tissue is less common, accounting for about 5–16% (16), and duodenum, colon or biliary mucosa are rare. In this case, the child had a large diverticulum (about 5 cm), with the presence of both gastric mucosa and pancreatic tissue. Based on the special affinity of 99TcmO4 to gastric mucosa, the radionuclide SPECT/CT imaging should have been positive, but the 99TcmO4-SPECT/CT imaging, abdominal imaging examination failed to indicate typical MD manifestations, resulting in diagnosis difficulties. This may be associated with massive gastrointestinal bleeding (4,5,7,8) and diverticular inversion. Endo et al. (17) found that radionuclide scanning imaging may not be effective in the diagnosis of MD with inversion, and the diagnostic rate of DBE for these cases was 100%. The nuclide is easily dispersed and difficult to concentrate in the case of massive hemorrhage of digestive tract. The diverticulum turned inward, the mouth of the diverticulum inverted to the side of the intestinal cavity, and the nuclide could not enter the interior of the diverticulum and could not be visualized.
Diverticular inversion is one of the causes of MD complicated with intussusception and is easily misdiagnosed as intestinal tumor. There are few reports of MD’s inversion, and most of them are individual cases. The cause of diverticula inversion is unknown, and the diverticula is not fixed on the mesentery or intestine. Some scholars believe that ulcers of the intestinal mucosa and ectopic tissues at the base of the diverticula can cause abnormal peristalsis of the small intestine and lead to the diverticula inversion, but some cases of diverticula inversion do not have mucosal ulcers and ectopic tissues (11,18). Mesenteric fat of MD is pulled into the center of the diverticulum when it is turned over in the small intestinal lumen, and when the diverticulum is inverted, it can cause not only intestinal obstruction, but also intussusception. The diagnosis of diverticulum can only be confirmed by examination of pathological specimens during or after intussusception. MD varus can be diagnosed by endoscopy, CT, MRE and other imaging methods. However, the inverted adipose tissue is easily confused with intestinal lipoma on imaging (11,19). The mucosa of the diverticulum is stretched and edema makes it look more like a tumor or polyp under the microscope, and inside is a layer of adipose tissue enclosed by mesothelial cells -adipose tissue of the mesentery, which is inside the serosal membrane, and adipose tissue of a true lipoma, which is located below the mucosa. In this case, the diverticulum was observed to be a mass under the double-balloon enteroscopy, and MRE examination suspected neoplastic lesions, which could not be clearly identified as a diverticulum. Instead, the diverticulum was the result of inward swelling and interlock with the surrounding normal intestinal cavity.
Therefore, for children with high suspicion of MD, 99TcmO4-SPECT/CT imaging can be performed first, and if the result is negative, it is necessary to be alert to the possibility of false negative results due to excessive eating and incomplete digestive tract emptying, acute stage of massive digestive tract bleeding, inflammation and edema in diverticulum, location of diverticulum near the bladder area, special diverticulum (diverticulum with no ectopic gastric mucosa or too small ectopic tissue, vagal diverticulum, and inverted diverticulum). For these patients, we can consider the DBE or surgical exploration according to the patient’s condition to further confirm the diagnosis.
Conclusions
Based on this case, we believe that 99TcmO4-SPECT/CT imaging indicated the distribution of developer in the unusual site, but it does not meet the diagnostic conditions of MD, and other relevant tests can be further performed. Multipoint sampling of diverticula or lesion site through DBE for pathological examination and microscopic observation can initially diagnose MD and its lesion scope; And locate the preoperative surgical scope, so as to reduce the possibility of postoperative recurrence. MD inversion is the cause of diverticular complications, and it is prone to false negative test results leading to misdiagnosis.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-102/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-102/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-102/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The study was reviewed and approved by Ethics Committee of Jiangmen Clinical College of Guangdong Medical University/Jiangmen Central Hospital (approval number: [2023]4). Written informed consent was obtained from the patient and his parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bejiga G, Ahmed Z. Gangrenous Meckel's diverticulum with small bowel obstruction mimicking complicated appendicitis: 'Case report'. Int J Surg Case Rep 2022;97:107419. [Crossref] [PubMed]
- Gomes GF, Bonin EA, Noda RW, et al. Balloon-assisted enteroscopy for suspected Meckel's diverticulum and indefinite diagnostic imaging workup. World J Gastrointest Endosc 2016;8:679-83. [Crossref] [PubMed]
- Hansen CC, Søreide K. Systematic review of epidemiology, presentation, and management of Meckel's diverticulum in the 21st century. Medicine (Baltimore) 2018;97:e12154. [Crossref] [PubMed]
- Devi GK, Goei AHY, Ragavendra K, et al. Meckel's Diverticulum - Clinical Presentation and Pitfalls in Diagnosis in the Pediatric Age Group in Singapore. J Indian Assoc Pediatr Surg 2022;27:340-4. [PubMed]
- McDonald JS, Horst KK, Thacker PG, et al. Meckel diverticulum in the pediatric population: Patient presentation and performance of imaging in prospective diagnosis. Clin Imaging 2022;91:37-44. [Crossref] [PubMed]
- Yan P, Jiang S. Tc-99m scan for pediatric bleeding Meckel diverticulum:a systematic review and meta-analysis. J Pediatr (Rio J) 2023;99:425-31. [Crossref] [PubMed]
- Spottswood SE, Pfluger T, Bartold SP, et al. SNMMI and EANM practice guideline for meckel diverticulum scintigraphy 2.0. J Nucl Med Technol 2014;42:163-9. [Crossref] [PubMed]
- Dolezal J, Vizda J. Experiences with detection of the ectopic gastric mucosa by means of Tc-99m pertechnetate disodium scintigraphy in children with lower gastrointestinal bleeding. Eur J Pediatr Surg 2008;18:258-60. [Crossref] [PubMed]
- Baltes P, Dray X, Riccioni ME, et al. Small-bowel capsule endoscopy in patients with Meckel's diverticulum: clinical features, diagnostic workup, and findings. A European multicenter I-CARE study. Gastrointest Endosc 2023;97:917-926.e3. [Crossref] [PubMed]
- Tanaka Y, Motomura Y, Akahoshi K, et al. Capsule endoscopic detection of bleeding Meckel's diverticulum, with capsule retention in the diverticulum. Endoscopy 2010;42:E199-200. [Crossref] [PubMed]
- Tang XB, Wang X, Ma Y, et al. Radiological and clinical characteristics of intussuscepted, inverting, and inverted Meckel's diverticulum: A case series. Eur J Radiol 2022;157:110611. [Crossref] [PubMed]
- Zhu Z, Zhou S, Cai H, et al. The diagnostic and treatment values of double-balloon enteroscopy in children's Meckel's diverticular bleeding. Medicine (Baltimore) 2021;100:e24823. [Crossref] [PubMed]
- He Q, Zhang YL, Xiao B, et al. Double-balloon enteroscopy for diagnosis of Meckel's diverticulum: comparison with operative findings and capsule endoscopy. Surgery 2013;153:549-54. [Crossref] [PubMed]
- Chang KC, Chang CH, Chou JW, et al. Meckel's diverticulum diagnosed by double-balloon enteroscopy: A single-center retrospective study in Taiwan. JGH Open 2022;6:63-8. [Crossref] [PubMed]
- Park JJ, Wolff BG, Tollefson MK, et al. Meckel diverticulum: the Mayo Clinic experience with 1476 patients (1950-2002). Ann Surg 2005;241:529-33. [Crossref] [PubMed]
- Xinias I, Mavroudi A, Fotoulaki M, et al. Wireless Capsule Endoscopy Detects Meckel's Diverticulum in a Child with Unexplained Intestinal Blood Loss. Case Rep Gastroenterol 2012;6:650-9. [Crossref] [PubMed]
- Endo Y, Jimbo K, Arai N, et al. A Pediatric Case of Inverted Meckel's Diverticulum Presenting with Cyclic Vomiting-like Symptoms: A Case Report and Literature Review. Children (Basel) 2022;9:1817. [Crossref] [PubMed]
- Blakeborough A, McWilliams RG, Raja U, et al. Pseudolipoma of inverted Meckel's diverticulum: clinical, radiological and pathological correlation. Eur Radiol 1997;7:900-4. [Crossref] [PubMed]
- El Hajra Martínez I, Calvo M, Martínez-Porras JL, et al. Inverted Meckel's diverticulum diagnosed using capsule endoscopy: A case report. World J Gastroenterol 2021;27:6154-60. [Crossref] [PubMed]
Cite this article as: Hu S, Du H, Wen J, Wu M, Huang B, Zhong J, Shi C, Liu C. Diagnosis of Inverted Meckel’s diverticulum by double-balloon enteroscopy: a case report. AME Case Rep 2024;8:33.


