
Resolving persistent air leaks associated with autosomal dominant hyper-IgE syndrome using one-way endobronchial valves: report of cases
Highlight box
Key findings
• Endobronchial valves (EBVs) are efficacious in resolving persistent air leaks (PALs) in autosomal dominant hyper-IgE syndrome (AD-HIES).
What is known and what is new?
• Patients with AD-HIES are at risk of developing PAL following development of bronchopleural fistula in the setting of recurrent lung infections.
• This manuscript demonstrates a safe, efficacious, and minimally-invasive method to resolve PAL in this population.
What is the implication, and what should change now?
• EBV placement should be considered when managing PAL in a patient with AD-HIES.
Introduction
The majority of cases of autosomal dominant hyper-IgE syndrome (AD-HIES) are caused by dominant negative mutations in STAT3. The gene product is a key protein involved in numerous biological pathways, including immunity and wound healing (1).
Starting early in life, AD-HIES patients experience recurrent Staphylococcus aureus (S. aureus), Streptococcus pneumoniae, and Haemophilus influenzae lung infections. These lung infections characteristically lack or show diminished systemic symptoms. Thus, delay in diagnosis is a common event and this situation culminates in the development of pneumatoceles or bronchiectasis (2). These may ultimately require surgical intervention. Subsequently, patients are at increased risk of secondary Gram-negative, mold, and non-tuberculous mycobacterial infections. Overall, morbidity and mortality are significant. Due to abnormal remodeling of lung tissue, approximately 50% of AD-HIES patients will develop a postoperative and prolonged bronchopleural fistula (BPF) (3). In this subgroup, definitive treatment has been difficult without additional surgery, which has led to more lung tissue loss, and further worsened their pulmonary compromise. Here, we report the utility of one-way endobronchial valves (EBV) in the management of BPFs in different scenarios of AD-HIES. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-35/rc).
Case presentation
The first patient is a 31-year-old male with well-established history of recurrent pneumonias, resultant pneumatoceles, and bronchiectasis. He presented with a chief complaint of fever and productive cough. His medical and genetic history is notable for AD-HIES, specifically a STAT3 mutation c.1144 C>T; p.R382W. The second patient is a 25-year-old male presenting with an increasing right upper lobe (RUL) pneumatocele in the context of AD-HIES, specifically STAT3 mutation c.1027 G>T; p.V343F.
The first patient had imaging of the lungs that showed dense RUL and left lower lobe (LLL) infiltrates associated with heavy growth of S. aureus and Pseudomonas aeruginosa (P. aeruginosa). Three days after admission for intravenous antibiotics, he developed acute onset shortness of breath and hypoxemia associated with a large right sided hydropneumothorax. Chest tube insertion drained the pleural fluid and expanded the lung. However, a significant air leak developed five days later. Its magnitude was so great that constant suction was required to maintain lung expansion. This situation evolved into a persistent air leak (PAL) over the next two weeks (Table 1). Informed consent was obtained under the National Institute of Allergy & Infectious Disease Review Board (identifier NCT00006150) approved device protocol to use EBV.
Table 1
| Patient No. | Presentation | Complication prompting EBV | EBV placement | Removal of chest tubes and EBV |
|---|---|---|---|---|
| Patient 1 | Date of presentation and initiation of antibiotics: hospital day 0 | Development of hydropneumothorax and resultant chest tube insertion: hospital day 3 | Air leak recurred: hospital day 5 | Removal of chest tube: hospital day 37 |
| Bronchoscopy and placement of 6 mm EBV: hospital day 19 | Removal of EBV: 21 days later | |||
| Patient 2 | Video-assisted thoracoscopic surgery with partial bullectomy: hospital day 0 | Development of hematoma, sanguineous chest tube output: hospital day 2 | EBV placement: Hospital day 14 | Chest tube removal: 12 days after final EBV placement, 7 weeks after initial presentation |
| Additional EBV placement: 6 weeks later |
EBV, endobronchial valve.
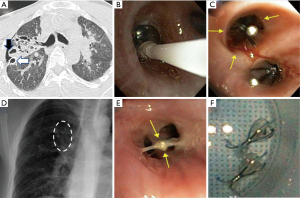
The patient underwent bronchoscopy under general anesthesia. An Olympus B5-2c balloon catheter was inserted into the right-side airways with sequential inflations at lobar and segmental bronchi to localize the air leak. A total of two 6-mm Spiration EBV (Olympus Respiratory America, Redmond, WA, USA) were placed in the posterior (B3) and apical (B1) subsegments of the RUL (Figure 1). Eighteen days after EBV placement, the patient’s chest tube was removed with no recurrence of pneumothorax. Intravenous antibiotics were then changed to oral antibiotics covering the infecting S. aureus and P. aeruginosa. Three weeks later, the valves were removed without complication.
The second patient underwent video-assisted thoracoscopic surgery (VATS) with partial bullectomy. Post-operatively, a hemothorax developed with sanguineous chest tube drainage. After the hematoma was evacuated, the patient continued to have a continuous PAL. After two weeks, informed consent was obtained under an IRB approved protocol for placement of EBV.
A 7-Fr Fogarty balloon catheter was used to serially occlude the orifices of the right sided airways as above, localizing the source of PAL in the apical lung region. Three 7-mm EBVs were inserted in the apical, posterior, and anterior segmental bronchi (B1–3) of RUL. After valve placement, the magnitude of the PAL decreased, but remained present. Subsequent upsizing of the posterior segmental bronchus (B3) EBV to 9 mm (due to improper seating) did not completely resolve the PAL. Doxycycline pleurodesis via retrograde instillation via the chest tube was also ineffective. Six weeks later, a second set of EBV was placed to occlude the right middle lobe (RML) segmental bronchi (B4–5) (two 7-mm valves in the lateral segmental bronchus B4 and a 9-mm valve in the medial segmental bronchus B5) and superior segmental bronchus (7-mm valve at B6) of right lower lobe (RLL). Within a few days, the magnitude of PAL was significantly diminished and chest imaging demonstrated improved lung expansion. The chest tube was removed 12 days afterwards. Intravenous antibiotics targeting the airway colonizing Escherichia coli and P. aeruginosa that were continued through the PAL were changed to oral suppression. At six weeks after the placement of the last set of valves, all EBVs were removed without complication.
All procedures performed in this study were in accordance with the ethical standards of the National Institute of Allergy & Infectious Disease Review Board (identifier NCT00006150), and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient consent according to the National Cancer Institute ethics committee/institutional review board and approved by the IRB.
Discussion
The consideration of EBV for therapeutic interventions of AD-HIES sequelae is important as these patients have a high rate of BPF post-surgery or are plagued by spontaneous pneumothorax from pneumatocele rupture. After lung damage or surgery, IL-6-STAT3 signaling promotes cilia formation in newly re-epithelialized lung tissue (4). Furthermore, STAT3 is involved in the regulation of matrix metalloproteinases, which are important for tissue remodeling and which have been associated with acute lung inflammation. This normal process is dysregulated in AD-HIES patients. PAL generally are associated with higher morbidity and mortality, longer chest tube duration, prolonged hospital stay, and higher resource utilization. In AD-HIES patients they frequently are associated with empyema, prolonged thoracostomy tube drainage, and secondary surgeries (3).
Many advantages are associated with use of EBV, including being minimally invasive and feasible to perform at any institution. From an economic perspective, EBV can minimize the length of hospital stays due to shorter duration of tube thoracostomy, which often requires suction to counter the lung collapsing effect of large magnitude BPF associated with PAL (4). Risks from EBV use include atelectasis, valve migration or expectoration, moderate oxygen desaturation, pneumothorax formation, and lung infection (4). However, few complications related to EBVs have been reported.
In our first patient, a PAL resulted from a hydropneumothorax after bacterial lung infection. Whereas in our second patient, the PAL formed after pneumatocele resection. Typical methods of treating BPF with fibrin, tissue glue, and/or doxycycline pleurodesis are seldom successful in AD-HIES patients. EBV, which have become widely accessible, are an underutilized intervention in this complex patient group. The EBV are umbrella-shaped and used to limit airflow one-way distally while allowing drainage of secretions proximally. Though originally designed for use in non-surgical lung volume reduction in chronic obstructive pulmonary disease (COPD), the Food and Drug Administration (FDA) in 2008 granted a humanitarian device exemption to EBV use in the treatment of PAL. Since then, EBV have been used with great success in patients with complicated PAL. Although, unlike the multiple patient trials that have defined the clinical scenarios for successful deployment of EBV in COPD (5,6), PAL physiology is different and not yet rigorously studied in EBV trials (6).
In two retrospective studies examining EBV use for the resolution of post-operative PAL, Travaline et al. and Reed et al. reported a 79% and 93% success rate, respectively (7,8). To our knowledge, ours is the first case cohort that describes the use of EBV for the management of PAL in AD-HIES patients. The EBV were well tolerated in these AD-HIES patients, and no progressive infections were noted, although suppression of the airway colonizing pathogens were continued while the EBV were in place due to the patients’ immune compromised state. Strengths of this report are the availability of the entirety of clinical information prior to and post-placement, including relevant genetic data. It also provided a brief overview of the utility of EBV in this unique and challenging patient population. Shortcomings include the description of only two cases.
Conclusions
This case report represents a description of available literature, references to relevant data, and a description of two cases in which EBV placement in AD-HIES was successful and without complication. It represents not only a description of the technique and its applicability, but also how to alter the surgical plan when initial placement requires upsizing and additional EBV, as demonstrated in the second patient. We illustrated that EBV can be useful in treating BPF that leads to PAL even in the setting of altered lung healing. Our experiences suggest that EBV should be considered in the first-line during management of PAL, but more prospective studies are needed in AD-HIES patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-35/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-35/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-35/coif). K.N.O was funded in part by the intramural research program of NHLBI, NIH (No. ZIA HL 006201) and through the NHLBI intramural/extramural (grant No. U01 HL 156655). C.D.H. was funded in part by the intramural research program of NCI, NIH (No. ZIA BC 011657). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from additional patient consent according to the National Institute of Allergy & Infectious Disease Review Board (No. NCT00006150).
Disclaimer: The article represents the opinion of the authors and does not represent the opinion of the United States Navy, DoD, or the United States Government.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 2007;357:1608-19. [Crossref] [PubMed]
- Freeman AF, Olivier KN. Hyper-IgE Syndromes and the Lung. Clin Chest Med 2016;37:557-67. [Crossref] [PubMed]
- Freeman AF, Renner ED, Henderson C, et al. Lung parenchyma surgery in autosomal dominant hyper-IgE syndrome. J Clin Immunol 2013;33:896-902. [Crossref] [PubMed]
- Tadokoro T, Wang Y, Barak LS, et al. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci U S A 2014;111:E3641-9. [Crossref] [PubMed]
- Li A, Lee P. Which Endoscopic Procedure to Use and in What Patient? Valves, Coils, Foam, and Heat in COPD and Asthma. Pulm Ther 2023;9:49-69. [Crossref] [PubMed]
- Mahajan AK, Khandhar SJ. Bronchoscopic valves for prolonged air leak: current status and technique. J Thorac Dis 2017;9:S110-5. [Crossref] [PubMed]
- Travaline JM, McKenna RJ Jr, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest 2009;136:355-60. [Crossref] [PubMed]
- Reed MF, Gilbert CR, Taylor MD, et al. Endobronchial Valves for Challenging Air Leaks. Ann Thorac Surg 2015;100:1181-6. [Crossref] [PubMed]
Cite this article as: Kucera J, Buhaya M, Sartain NN, Olivier KN, Freeman AF, Hoang CD. Resolving persistent air leaks associated with autosomal dominant hyper-IgE syndrome using one-way endobronchial valves: report of cases. AME Case Rep 2024;8:43.


