
Advanced gallbladder cancer with high tumor mutation burden: a case report and literature review
Highlight box
Key findings
• A patient with advanced gallbladder cancer (GBC) and high tumor mutation burden (TMB) benefited from first-line treatment with mFOLFIRINOX.
• After undergoing sequential chemotherapy with immunotherapy, the patient experienced tumor recurrence and metastasis.
What is known and what is new?
• Combining chemotherapy with immunization enhances the first-line treatment of advanced GBC.
• The timing of combination therapy needs to be carefully selected as the immunotherapy may be best employed at an early stage.
What is the implication, and what should change now?
• The use of immunotherapy in combination with chemotherapy may not be appropriate in advanced stages with high TMB.
• More clinical trials involving immunotherapy in GBC are appealed to identify biomarkers that can guide clinical decisions for a new era of individualized therapy.
Introduction
Background
Biliary tract cancers (BTCs) are a heterogeneous group of aggressive, rare malignant tumours that originate in the bile ducts, both within and outside the liver (1). BTCs comprise cholangiocarcinoma, gallbladder cancer (GBC), and ampulla of Vater cancer (AVC) (2). GBC is a malignant tumor of the biliary system, accounting for approximately 90% of BTCs (3-6). GBC has an annual incidence rate of 1.5 to 2.7 cases per 100,000 people (7-9), making it the sixth most common gastrointestinal cancer. It is an aggressive malignant tumor with a 5-year survival rate ranging from 4% to 60%, depending on the stage and resectability of the tumor (10-14). For patients with GBC, negative surgical margins are still the only hope for long-term survival. Unfortunately, most GBCs are diagnosed at an advanced stage, and 40–75% of patients diagnosed have metastatic disease (15). Advanced, unresectable and metastatic GBC is associated with a poor prognosis and systemic chemotherapy is the only treatment option for these patients (16). Currently, platinum or fluorouracil are the most commonly used chemotherapeutic agents for advanced GBC. However, an alternative chemotherapy regimen combining gemcitabine and cisplatin (CISGEM) was proposed in the ABC-02 clinical trial for BTCs, including locally advanced or metastatic cholangiocarcinoma, ampullary carcinoma and GBC (17-19). The ABC-02 and BT22 trials have revolutionised first-line treatment and established gemcitabine and cisplatin (CISGEM) as the new standard of care for advanced disease (19,20). The mFOLFIRINOX regimen and the CISGEM regimen have shown favorable activity and tolerability in retrospective and phase II clinical trials in first and/or second line treatment of advanced BTC (21). Results from the phase III TOPAZ-1 trial showed that immunotherapy in combination with CISGEM improved overall survival (OS), suggesting that immunotherapy has significant clinical benefit for cancer patients. However, there are only a few reports of patients with advanced GBC who have received sequential chemotherapy with immunotherapy after first-line chemotherapy. The modest survival benefit of dual agents suggests that there is an urgent need to define new first-line strategies for the treatment of metastatic BTC (22). Here, we report a case of a patient with high cc (TMB) advanced GBC who benefited from first-line treatment with mFOLFIRINOX and underwent sequential chemotherapy with immunotherapy followed by tumor recurrence and metastasis and death, which may provide a reference for clinical optimisation of treatment modalities for patients with advanced GBC. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-188/rc).
Case presentation
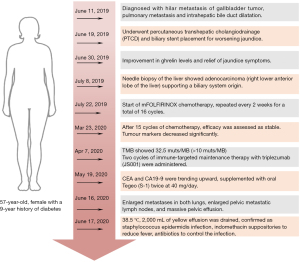
Here, we report the case of a 57-year-old woman with a 9-year history of diabetes. A timeline summarizing the main events of this case report is shown in Figure 1. On 11 June 2019, she developed yellow skin and sclera, dark-colored urine, loss of appetite, fatigue, and abdominal distension, which worsened after meals. These symptoms were accompanied by tolerable back and right upper abdominal pain and nausea without vomiting. An upper abdominal computed tomography (CT) scan was performed in the outpatient clinic and showed an obstruction in the area of the hepatic portal vein. Liver function tests showed elevated levels of glutamic-pyruvic transaminase: 152 U/L (normal: 7–40 U/L), glutamic-oxaloacetic transaminase: 90 U/L (normal: 13–35 U/L), total bilirubin: 244.5 µmol/L (normal: 5–21 µmol/L), direct bilirubin: 219.8 µmol/L (normal 0–6 µmol/L), and indirect bilirubin: 24.7 µmol/L (normal: 2–15 µmol/L). Following an enhanced abdominal CT scan, she was diagnosed with “hilar metastasis of gallbladder tumor, pulmonary metastasis and intrahepatic bile duct dilatation”. On 19 June 2019, the patient underwent percutaneous transhepatic cholangiodrainage (PTCD) and biliary stent placement for worsening jaundice. Post-operative catheter drainage was uneventful. Eleven days later, liver function tests showed improved glutamic-pyruvic transaminase levels: 43 U/L, glutamic-oxaloacetic transaminase: 35 U/L, total bilirubin: 80.2 µmol/L, direct bilirubin: 75.3 µmol/L, and indirect bilirubin: 4.9 µmol/L. Jaundice symptoms were also relieved.
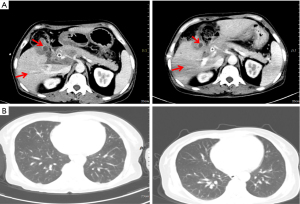
On 8 July 2019, an ultrasound-guided needle biopsy of the liver was performed. The results confirmed an adenocarcinoma (of the right lower anterior lobe of the liver), which supported the origin of the bile duct system. Immunohistochemistry results were: carbohydrate antigen 19-9 (CA19-9) (+), CD34 (−), CK19 (+), CK7 (+), Heppar-1 (−), KI-67 (20%), β-catenin (−), CK8/18 (+), CK20 (−), and Villin (+). Imaging and pathology are shown in Figures 2,3, respectively. The results of gene detection by puncture pathology showed that the TMB was 32.5 muts/MB, including three mutations were in critical genes (Table 1).
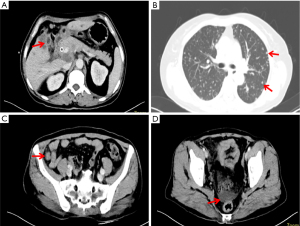
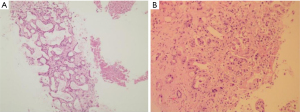
Table 1
| Important mutations variation | Mutation rate of abundance/copy |
|---|---|
| TP53: NM_000546: exon8: c.886delC: p.H296fs, % | 25.20 |
| ERBB2: NM_004448: copy number, % | 2.83 |
| IDH1: NM_005896: exon4: c.C395T: p.R132H | 1.00 |
| TMB, muts/MB | 32.50 |
| Objective response rate, % | 36.90 |
TMB, tumor mutation burden.
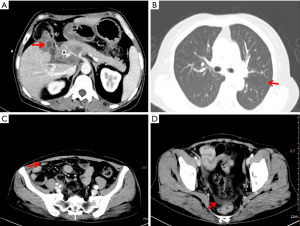
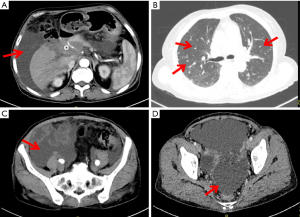
On 22 July 2019, she was prescribed mFOLFIRINOX, a 4-drug combination regimen. On the first day of each cycle, oxaliplatin 85 mg/m2 [intravenous (IV), 120 min], irinotecan 150 mg/m2 (IV, 90 min), leucovorin 400 mg/m2 (IV, 2 h, at the same time as irinotecan), and 5-fluorouracil (5-FU) 2,400 mg/m2 (IV for 46 h), were administered and repeated every two weeks. A total of 16 cycles of the chemotherapy regimen were administered. Imaging evaluation after six cycles of chemotherapy showed a partial response (PR) and a significant reduction in lung metastases and bilateral iliac fossa nodules (Figure 4). After 10 cycles and 15 cycles of chemotherapy, the patients were re-evaluated with enhanced CT of the chest and abdomen, and were found to be in stable condition (Figure 5), with tumor markers such as CA19-9 and carcinoembryonic antigen (CEA) decreasing significantly. Patient’s cough and sputum also showed significant improvement, and her weight increased by 10 kg.
Notably, the patient experienced some significant side effects during chemotherapy and related treatments. The patient developed mild tongue numbness and sporadic abdominal discomfort on the first day after the first cycle of chemotherapy, which may be due to the neurotoxicity of oxaliplatin. The patient also developed delayed diarrhoea with watery stools, which was treated with oral Imodium. A blood test on admission prior to the ninth cycle of chemotherapy showed grade II post-chemotherapy myelosuppression, and pegylated recombinant human granulocyte stimulating factor (rhG-CSF) was administered.
However, after the last cycle of mFOLFIRINOX chemotherapy (cycle 16), the patient felt weak and was unable to tolerate systemic intravenous chemotherapy. The gene puncture test showed that the patient had a TMB of 32.5 muts/MB (≥10 muts/MB). Two cycles of immune-targeted maintenance therapy with triplezumab (JS001) were administered from 7 to 28 April 2020. During the second cycle of immunotherapy, the tumor blood markers CEA and CA19-9 showed an increasing trend, so the second cycle was supplemented with two oral treatments of 40 mg of S-1 per day.
Noteworthily, on 16 June 2020, an imaging study showed an increase in metastatic tumors in both lungs, enlarged metastatic lymph nodes in the pelvic cavity and a large amount of fluid in the pelvic cavity (Figure 6). The patient received a percutaneous peritoneal fluid puncture for drainage and approximately 2,000 mL of yellow fluid was drained. During the drainage procedure she developed a fever with a body temperature of 38.5 ℃. The fever was reduced with an indomethacin suppository. Microbiological cultures confirmed the presence of staphylococcus aureus in the ascites. Antibiotics (levofloxacin) were given according to the sensitivity of the bacterial culture. In addition, given the progression of the tumor, discontinuation of immunotherapy was recommended. The patient chose to be discharged from hospital and eventually died at home.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s family member for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
GBC is the most common type of BTC. It is usually diagnosed at an advanced stage, which leads to a poor prognosis and limited treatment options. The CISGEM regimen is the reference first-line chemotherapy for patients with advanced BTC (17). It is also recommended as first-line treatment for unresectable and metastatic BTCs, including unresectable GBC. Meanwhile, FOLFIRINOX is currently applied in the post-operative adjuvant treatment of pancreatic ductal adenocarcinoma (PDAC) and advanced metastatic pancreatic cancer. The FOLFIRINOX regimen was opted, because of the similarities in histology, treatment, and prognosis between BTCs and pancreatic adenocarcinoma (PA). Then, the patient experienced higher grade III/IV toxicity, particularly neutropenia, diarrhoea and peripheral neuropathy, similar to a landmark study by Conroy et al. (23). To reduce toxicity, mFOLFIRINOX protocols with reduced irinotecan or 5-FU doses are commonly used (24).
Over the past decade, immunotherapy has changed the treatment paradigm for a wide range of solid tumors, improving clinical outcomes and achieving unprecedented response rates (25,26). The OS benefit was more pronounced in the intrahepatic cholangiocarcinoma (iCCA) subgroup of the KEYNOTE-966 study, which may be due to the fact that these patients were not inherently sensitive to CISGEM and were more sensitive to immunotherapy. MOUSEION-01 study shows that women who receive single-agent immunotherapy benefit less than men who receive placebo (27). The recent MOUSEION-06 study showed that checkpoint inhibitor monotherapy or immune-based combination therapy was associated with improved survival regardless of whether the ECOG PS was 0 or 1. We need real-world clinical trials with more patients (28). In current clinical practice, there are a number of cases where patients with GBC have achieved a survival benefit after receiving immunotherapy, which means that there must be patients with GBC who can benefit from the survival benefit of immunotherapy. More immunotherapy trials for bile duct cancer are underway.
Genetic testing showed that the patient had a TMB of up to 32.5 muts/MB (TMB ≥10 muts/MB). Samstein et al. showed that TMB and OS are positively correlated, suggesting that TMB can predict the clinical response to immune checkpoint inhibitors (ICIs) (29). A meta-analysis found a significant correlation between TMB and objective response rate (ORR) in 27 different tumors, including GBC (30). In the KEYNOTE-158 study, patients with TMB-H (≥10 muts/MB) had a higher ORR with pembrolizumab than patients with TMB <10 muts/MB (29% vs. 6%) across 10 tumors, including anal canal and BTC (31). The TOPAZ-1 trial (NCT03875235) represents a breakthrough in the first-line treatment of advanced BTC with a combination approach of immunotherapy and chemotherapy. In addition, a phase I clinical trial conducted in Japan found that the ICI (nivolumab), either alone or in combination with cisplatin + gemcitabine chemotherapy, had a manageable safety and efficacy profile and that first-line immunotherapy in combination with chemotherapy was more effective (32). Patients with advanced tumors who cannot receive standard treatment may benefit from immunotherapy if they have a high TMB. However, TMB is not unanimously accepted (33,34). The CheckMate 227 study updates OS data and finds that TMB does not predict OS gain. In the KEYNOTE-021/189/407 study, there was no correlation between TMB and the efficacy of pembrolizumab + chemotherapy. In conclusion, TMB may predict response to ICI therapy in some cases, but conclusions are inconsistent, and caution should be exercised, especially when TMB predicts long-term outcomes and the efficacy of combination immunotherapy. Combining multiple biomarkers, such as programmed cell death-ligand 1 (PD-L1) and TMB, may be a better way to screen for immunotherapy benefit. Other immunotherapy trials for GBC are underway.
Toripalimab is approved for the second-line treatment of unresectable or metastatic malignant melanoma following failure of prior systemic therapy, recurrent or metastatic nasopharyngeal carcinoma and metastatic urothelial carcinoma following failure of prior systemic therapy. Given the patient’s financial situation, we administered toripalimab (JS001) for 1 cycle. In addition, the oral fluorouracil derivative S-1, with or without gemcitabine, is considered a promising treatment for advanced GBC. In the Japanese randomized phase II study JCOG 0805 (35), the median progression-free survival (PFS) with S-1 monotherapy was 4.2 months. Due to the increased trend of CEA and CA19-9 prior to immunotherapy in the second cycle and the frail physical condition of the patients, S-1 was included in the therapeutic regimen. Unfortunately, after two months of immunotherapy, the patient had massive fluid accumulation in the abdominal cavity and abnormal levels of tumor markers. Percutaneous peritoneal effusion puncture drainage was performed to drain the ascites. During the drainage, antibiotics were given to reduce the abdominal infection.
Based on the findings of Ueno et al. (32) and our case, we speculate that immunotherapy combined with chemotherapy may be more effective for first-line use in advanced GBC with high TMB. Maybe we should try to use immunotherapy as early as possible. In addition, our patient was weak and susceptible to infection after receiving chemotherapy, which was exacerbated by the combination of antibiotics and immunotherapy, which may have contributed to the failure of our treatment. The duration of antibiotic exposure is an important influence on the relationship between antibiotics and ICB response. Whether patients’ OS and PFS would be affected after the combination of antibiotics and immunotherapy was an open question that needs to be validated in a large sample of clinical trials to further assess the efficacy of these combination regimens. A 2019 meta-analysis reported longer OS and PFS in patients who were not exposed to antibiotics during immune checkpoint lockdown blockade (ICB) (36). It has been reported that the use of antibiotics 42 days before starting ICB appears to be harmful (36). Of note, the heterogeneity in terms of mutational burden and poor prognosis in patients with GBC should be taken into account, as the use of immunotherapies such as ICB can cause hyper-progressive disease (HPD) (37). Therefore, further studies are needed to determine the curative effect of immunotherapy in GBC and the molecular mechanisms involved in transformation to HPD.
Conclusions
In conclusion, we report the case of an advanced GBC patient treated with an mFOLFIRINOX chemotherapy whose PFS and OS were significantly prolonged with tolerable side effects. Further sequential immunotherapy in the patient had a poor effect, suggesting that the use of immunotherapy in combination with chemotherapy at a later stage may be inappropriate and that the timing of combination therapy needs to be carefully chosen. Therefore, more clinical trials of immunotherapy in GBC are needed to identify biomarkers to guide clinical decisions and open a new chapter in individualized therapy.
Acknowledgments
We thank the Departments of Pathology, Invasive Technology, and Hepatology, Qilu Hospital (Qingdao) for their support in the collection of pathological and clinical data.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-188/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-188/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-188/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat Res Commun 2021;27:100354. [Crossref] [PubMed]
- Ricci AD, Rizzo A, Brandi G. Immunotherapy in Biliary Tract Cancer: Worthy of a Second Look. Cancer Control 2020;27:1073274820948047. [Crossref] [PubMed]
- Bridgewater JA, Goodman KA, Kalyan A, et al. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am Soc Clin Oncol Educ Book 2016;35:e194-203. [Crossref] [PubMed]
- Van Dyke AL, Shiels MS, Jones GS, et al. Biliary tract cancer incidence and trends in the United States by demographic group, 1999-2013. Cancer 2019;125:1489-98. [Crossref] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [PubMed]
- Huang J, Lucero-Prisno DE 3rd, Zhang L, et al. Updated epidemiology of gastrointestinal cancers in East Asia. Nat Rev Gastroenterol Hepatol 2023;20:271-87. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012;6:172-87. [Crossref] [PubMed]
- Kanthan R, Senger JL, Ahmed S, et al. Gallbladder Cancer in the 21st Century. J Oncol 2015;2015:967472. [Crossref] [PubMed]
- Moris D, Tsilimigras DI, Lim J, et al. Neuroendocrine carcinomas of the gallbladder: Lessons learnt from cases at opposite ends of the spectrum. J BUON 2018;23:1922-6. [PubMed]
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg 2000;232:557-69. [Crossref] [PubMed]
- Dixon E, Vollmer CM Jr, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg 2005;241:385-94. [Crossref] [PubMed]
- Singh SK, Talwar R, Kannan N, et al. Patterns of Presentation, Treatment, and Survival Rates of Gallbladder Cancer: a Prospective Study at a Tertiary Care Centre. J Gastrointest Cancer 2018;49:268-74. [Crossref] [PubMed]
- Tsilimigras DI, Hyer JM, Paredes AZ, et al. The optimal number of lymph nodes to evaluate among patients undergoing surgery for gallbladder cancer: Correlating the number of nodes removed with survival in 6531 patients. J Surg Oncol 2019;119:1099-107. [Crossref] [PubMed]
- Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-9. [Crossref] [PubMed]
- Azizi AA, Lamarca A, Valle JW. Systemic therapy of gallbladder cancer: review of first line, maintenance, neoadjuvant and second line therapy specific to gallbladder cancer. Chin Clin Oncol 2019;8:43. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Tsukiyama I, Ejiri M, Yamamoto Y, et al. A Cost-Effectiveness Analysis of Gemcitabine plus Cisplatin Versus Gemcitabine Alone for Treatment of Advanced Biliary Tract Cancer in Japan. J Gastrointest Cancer 2017;48:326-32. [Crossref] [PubMed]
- Rizzo A, Brandi G. First-line Chemotherapy in Advanced Biliary Tract Cancer Ten Years After the ABC-02 Trial: "And Yet It Moves!". Cancer Treat Res Commun 2021;27:100335. [Crossref] [PubMed]
- Rizzo A, Ricci AD, Tober N, et al. Second-line Treatment in Advanced Biliary Tract Cancer: Today and Tomorrow. Anticancer Res 2020;40:3013-30. [Crossref] [PubMed]
- Phelip JM, Desrame J, Edeline J, et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients With Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J Clin Oncol 2022;40:262-71. [Crossref] [PubMed]
- Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol 2019;30:1950-8. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Lambert A, Gavoille C, Conroy T. Current status on the place of FOLFIRINOX in metastatic pancreatic cancer and future directions. Therap Adv Gastroenterol 2017;10:631-45. [Crossref] [PubMed]
- Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: Looking outside the box. J Hepatol 2020;72:342-52. [Crossref] [PubMed]
- Reck M, Heigener D, Reinmuth N. Immunotherapy for small-cell lung cancer: emerging evidence. Future Oncol 2016;12:931-43. [Crossref] [PubMed]
- Santoni M, Rizzo A, Mollica V, et al. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol 2022;170:103596. [Crossref] [PubMed]
- Mollica V, Rizzo A, Marchetti A, et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med 2023;23:5039-49. [Crossref] [PubMed]
- Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202-6. [Crossref] [PubMed]
- Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017;377:2500-1. [Crossref] [PubMed]
- Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353-65. [Crossref] [PubMed]
- Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol 2019;4:611-21. [Crossref] [PubMed]
- Li R, Han D, Shi J, et al. Choosing tumor mutational burden wisely for immunotherapy: A hard road to explore. Biochim Biophys Acta Rev Cancer 2020;1874:188420. [Crossref] [PubMed]
- Zhang Y, Wang L, Li R, et al. The emerging development of tumor mutational burden in patients with NSCLC. Future Oncol 2020;16:469-81. [Crossref] [PubMed]
- Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci 2013;104:1211-6. [Crossref] [PubMed]
- Wilson BE, Routy B, Nagrial A, et al. The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Cancer Immunol Immunother 2020;69:343-54. [Crossref] [PubMed]
- Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol 2018;4:1543-52. [Crossref] [PubMed]
Cite this article as: Wang J, Liu J, Yan C, Wang K, Li Q, Yu J. Advanced gallbladder cancer with high tumor mutation burden: a case report and literature review. AME Case Rep 2024;8:53.


