
COVID-19 infection with severe hypocalcaemia and superior mesenteric artery syndrome—a case report
Highlight box
Key findings
• A young male who was admitted for coronavirus disease 2019 (COVID-19) infection also presented with concurrent symptomatic hypocalcaemia, as well as superior mesenteric artery syndrome from malnourishment. There were no features of chronic hypoparathyroidism in this patient, ruling out congenital causes of hypoparathyroidism and further strengthening the suspicion of COVID-19 as the cause of his permanent primary hypoparathyroidism.
What is known and what is new?
• Acute COVID-19 infections have been associated to various transient or permanent endocrinopathies, with several literature reporting transient hypocalcaemia or acute hypocalcaemic exacerbation in known hypoparathyroidism patients.
• This case report is the first to report persistent hypoparathyroidism as a sequela of a COVID-19 infection.
What is the implication, and what should change now?
• Further studies are required to determine the effects of severe acute respiratory syndrome coronavirus 2 infection on the parathyroid glands and its pathophysiology.
Introduction
Primary hypoparathyroidism secondary to coronavirus disease 2019 (COVID-19) is an uncommon presentation. Till date there have only been three cases reported in the literature so far (1-3). We report a case of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive young gentleman who presented symptomatic hypocalcaemia secondary to primary hypoparathyroidism, which may have complicated his superior mesenteric artery (SMA) syndrome. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-106/rc).
Case presentation
We hereby report a previously well 14-year-old gentleman who presented with hypercalcaemic symptoms, i.e., generalised tonic-clonic seizures, carpopedal spasms, perioral numbness, and bilateral hand numbness. Prior to these symptoms, he has had 4 days of fever with multiple episodes of vomiting and abdominal pain. The patient was from an impoverished family and has not been receiving adequate nutrition for the past few months, resulting in a significant history of weight loss. His younger brother and he are currently living with his divorced father, who has financial difficulty in providing adequate food for them. The patient is still schooling and has average academic achievement with no difficulty in school. Clinically he was dehydrated with a distended abdomen and sluggish bowel sounds. His body weight was merely 32.9 kg (<5th centile in growth chart for boys age 2–20 years) whilst his height was 153 cm (between 10th and 25th centile in growth chart for boys age 2–20 years), hence his body mass index was 14.1 kg/m2. Otherwise, aside from fever, his vital signs were normal and there were no clinical features suggestive of pseudohypoparathyroidism.
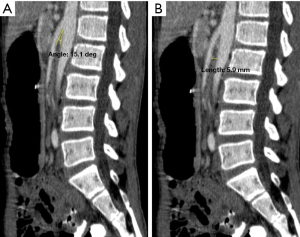
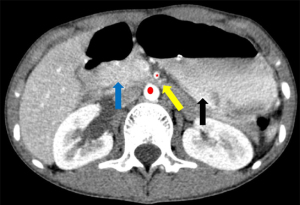
Blood investigations showed severe hypocalcaemia and hyperphosphatemia with an inappropriately lowish serum intact parathyroid hormone (iPTH) levels, suggesting a primary hypoparathyroidism with vitamin D insufficiency (serum 25-hydroxyvitamin D levels were 54.41 nmol/L) (Table 1). There was also prolonged corrected QT interval (QTc) interval in the electrocardiography (ECG) whilst his nasopharyngeal SARS-CoV-2 rapid antigen test was positive. His chest X-ray was normal whilst an urgent contrasted computed tomography scan of the abdomen suggested SMA syndrome (Figures 1A,1B,2).
Table 1
| Parameters | Day 1 (admission) | Day 40 (discharge) | Day 80 (review) |
|---|---|---|---|
| Body weight (kg) | 32.9 | 37.3 | 39.0 |
| Haemoglobin (normal range, 13.5–17.4) (g/dL) | 14.5 | 9.8 | 11.3 |
| Haematocrit (normal range, 40.1–50.6) (%) | 43.5 | 30.2 | 34.3 |
| Total white cell (normal range, 4.078–11.37) (×109/L) | 8.1 | 9.4 | 7.3 |
| Platelet (normal range, 142–350) (×109/L) | 179 | 221 | 163 |
| Urea (normal range, 3.2–8.2) (mmol/L) | 9.6 | 3.7 | 1.8 |
| Creatinine (normal range, 49–115) (μmol/L) | 103 | 58 | 51 |
| Corrected calcium (normal range, 2.08–2.65) (mmol/L) | 0.89 | 2.34 | 2.24 |
| Magnesium (normal range, 0.66–1.07) (mmol/L) | 0.75 | 0.62 | 0.73 |
| Phosphate (normal range, 0.78–1.65) (mmol/L) | 3.30 | 2.16 | 1.91 |
| Alkaline phosphatase (normal range, 46–116) (U/L) | 225 | 151 | 151 |
| Iron (normal range, 9.0–31.3) (μmol/L) | 5.2 | – | – |
| iPTH (normal range, 14.9–56.9) (pg/mL) | 14.7 | – | 5.5 |
| 24-hour urine calcium (normal range, 2.5–7.5) (mmol/d) | 11.73 | – | – |
| Anti-thyroid peroxidase Ab (normal range, <9) (IU/mL) | 0.39 | – | – |
| 25-hydroxy-vitamin D (normal range, 50–80) (nmol/L) | 54.41 | – | – |
iPTH, intact parathyroid hormone; Ab, antibody.
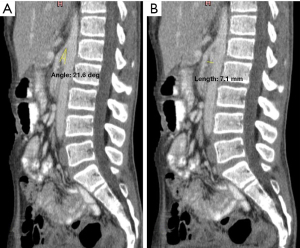
He was treated as category 2 COVID-19 infection with severe hypocalcaemia and SMA syndrome. The calcium levels were promptly corrected, and the patient was commenced with total parenteral nutrition since he was unable to tolerate oral feeding. Interestingly, with the correction of his calcium levels, his gastrointestinal symptoms dramatically improved. Eventually, after 40 days hospital stay with medical nutrition therapy, as well as calcium and calcitriol replacement, the patient was able to tolerate orally and hence uneventfully discharged with a body weight of 37.3 kg. During his post-discharge review (day 80 from discharge), a computed tomography scan of his abdomen was repeated, showing improvement in his SMA syndrome (Figure 3A,3B) The patient’s body weight had increased till 39 kg and still had primary hypoparathyroidism, requiring both oral calcium and calcitriol replacement.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s father for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
SMA syndrome, a rare cause of small bowel obstruction, happens due to a compression of the duodenal segment between SMA and abdominal artery, often by loss of the retroperitoneal mesenteric fat pad that keeps these structures apart. The normal angle between the small mesenteric artery and the aorta is often between 38° and 65° and any reduction of this angle causes the SMA syndrome (4,5). SMA syndrome often occurs in unwell individuals with extreme weight loss, resulting in the loss of the mesenteric fat pad (6). This syndrome is usually seen in older children and adolescents (7).
Classically, patients with SMA syndrome present with features of bowel obstruction, which are abdominal pain and distension, vomiting and obstipation. A plain radiograph often shows evidence of proximal obstruction, whilst a barium study may demonstrate an abrupt narrowing of the third part of the duodenum with obstruction. The aortomesenteric angle can be measured by ultrasonography (4), or by contrast-enhanced computed tomography scan. Often the diagnosis is made when there is a presence of an aortomesenteric angle <22° and the aortomesenteric distance <8–10 mm (7,8).
Treatment options include nutritional support for adequate weight gain and regrowth of the mesenteric fat pad and subsequently improvement in the aortomesenteric angle (9). Symptomatic relief can be opted with postural therapy (prone, knee-chest or left lateral position) or placement of a nasogastric tube, which aids by restoring the aortomesenteric distance, allowing enteral feeding and subsequently weight gain, and eventually relieving the obstruction (5,9). Finally, surgical intervention such as gastrojejunostomy, duodenojejunostomy, or division of the ligament of Treitz can be performed in severe cases with failure of conservative management (8).
We believe that the SMA syndrome in our patient is a result of malnourishment due to his impoverished family as he was severely underweight during admission. His symptoms improved with correction of the hypocalcaemia, as well as weight gain with medical nutrition therapy.
On the other hand, the severe hypocalcaemia as a complication of the COVID-19 infection in our patient might have exacerbated his SMA syndrome. Ca2+-sensing receptors have been discovered in intestinal epithelial cells, and are postulated to play a vital role in modulating intestinal motility (10,11). It is well known that hypercalcaemic states often present with constipation or other gastrointestinal complaints, but due to its critical role in muscle and nerve conduction, there is a possibility that severe hypocalcaemia may present with gastrointestinal symptoms (12).
Hypocalcaemia has been associated with severe COVID-19 infection (13), but the exact mechanism is still unclear. Inflammatory responses during COVID-19 infection may lead to various forms of endocrine tissue damage, including the parathyroid glands (3). The SARS-CoV-2 virus may damage the parathyroid glands by binding to the angiotensin-converting enzyme 2 (ACE2) receptors on acidophilic cells of the glands (3,14). Besides that, a relative hypoparathyroidism may occur due to chronic respiratory alkalosis, a sequela of raised respiratory effort, resulting in resistant PTH receptors to PTH hormones (14). However, this is unlikely in our patient, as the hypocalcaemia and hypoparathyroidism persisted despite him having recovered from the COVID-19 infection with no respiratory complications.
Prior to this, there had been three case reports on SARS-CoV-2 infection induced primary hypoparathyroidism (1-3), as well as several case reports on decompensation of existing primary hypoparathyroidism during COVID-19 infection (Table 2).
Table 2
| Parameters | Elkattawy et al., 2020 (1) | Dianatfar et al., 2021 (2) | Georgakopoulou et al., 2022 (3) | Current report |
|---|---|---|---|---|
| Age (years) | 46 | 44 | 53 | 14 |
| Sex | M | F | M | M |
| Presentation | Incidental finding | Tonic-clonic seizure, depressed mood | Incidental finding | Tonic-clonic seizures with carpopedal spasms, perioral numbness, and distal limb tingling |
| Course of COVID-19 infection | Critical | Severe | Severe | Mild |
| Onset | Late (2nd month of hospitalization) | Late (40 days after COVID-19 infection) | Early (during presentation of acute COVID-19 infection) | Early (during presentation of acute COVID-19 infection) |
| iPTH level [normal range] (pg/mL) | 8–10 [12–88] | <3 [11–67] | 11.7 [12–65] | 14.7 [14.9–56.9] |
| 25-OH vitamin D level [normal range] (ng/mL) | 7 [30–100] | 33.1 [30–100] | 38.4 [30–100] | 54.41 [30–100] |
| Magnesium [normal range] (mg/dL) | 1.9 [1.8–2.6] | 2 [1.8–2.6] | Not mentioned | 1.9 [1.8–2.6] |
| Calcium [normal range] (mg/dL) | 9.2 [8.5–11] | 6.2 [8.5–11] | 6.9 [8.6–10.2] | 3.6 [8.6–10.3] |
| Phosphorus [normal range] (mg/dL) | 6.9 [3.4–4.5] | 5.7 [2.7–4.5] | 4.7 [2.5–4.5] | 12.6 [3.4–4.5] |
| Albumin [normal range] (g/L) | 2.9 [3.5–5.5] | 3.9 [3.5–5.5] | 3.74 [3.5–5.0] | 3.12 [3.5–4.5] |
| Outcome | Recovery | Recovery | Recovery | Requires calcium and calcitriol supplementation |
COVID-19, coronavirus disease 2019; M, male; F, female; iPTH, intact parathyroid hormone.
We believe that our patient only developed acute hypoparathyroidism recently as there were no features of chronic hypoparathyroidism, such as bilateral cataracts or presence of intracerebral calcifications on computed tomography scan of the brain. He also had normal developmental milestones as a child with an average academic performance in school, with no obvious neuropsychiatric abnormalities. There were no other features of autoimmune diseases, but we were unable to completely rule out autoimmune hypoparathyroidism as we could not send for an anti-calcium sensing receptor antibody test. Furthermore, unlike the other cases, our patient may be suffering from permanent hypoparathyroidism as his serum iPTH levels were still severely low, requiring calcium and calcitriol supplementation, despite more than 2 months of recovery from the COVID-19 infection.
We have excluded other causes of primary hypoparathyroidism, such as iatrogenic, autoimmune diseases, infiltrative diseases, or any complex genetic defects. Hence, we conclude that our patient may have developed permanent hypoparathyroidism as a sequela of COVID-19 infection. Till date, our patient remains the first patient reported with permanent hypoparathyroidism from a severe COVID-19 infection.
Patient’s perspective
The patient and his guardian were surprised when being explained that the underlying acute endocrinal dysfunction might be a sequela of the acute COVID-19 infection. Nonetheless, they were relieved that his condition improved tremendously with proper nutrition and adequate calcium and vitamin D supplementation.
Conclusions
Parathyroid gland involvement in a COVID-19 infection is rare but not impossible. Further studies are needed to determine the mechanism and extent of damage of SARS-CoV-2 to the parathyroid glands.
Acknowledgments
We would like to acknowledge all medical personnel involved in the care of this patient.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-106/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-106/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-106/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s father for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elkattawy S, Alyacoub R, Ayad S, et al. A Novel Case of Hypoparathyroidism Secondary to SARS-CoV-2 Infection. Cureus 2020;12:e10097. [Crossref] [PubMed]
- Dianatfar M, Sanjari M, Dalfardi B. Hypoparathyroidism after COVID-19 pneumonia. Shiraz E-Medical Journal 2021;22:e115832. [Crossref]
- Georgakopoulou VE, Avramopoulos P, Papalexis P, et al. COVID-19 induced hypoparathyroidism: A case report. Exp Ther Med 2022;23:346. [Crossref] [PubMed]
- Reece K, Day R, Welch J. Superior Mesenteric Artery Syndrome with Abdominal Compartment Syndrome. Case Rep Emerg Med 2016;2016:7809281. [Crossref] [PubMed]
- Zaraket V, Deeb L. Wilkie's Syndrome or Superior Mesenteric Artery Syndrome: Fact or Fantasy? Case Rep Gastroenterol 2015;9:194-9. [Crossref] [PubMed]
- Sahni S, Shiralkar M, Mohamed S, et al. Superior Mesenteric Artery Syndrome: The Dark Side of Weight Loss. Cureus 2017;9:e1859. [Crossref] [PubMed]
- Gozzo C, Giambelluca D, Cannella R, et al. CT imaging findings of abdominopelvic vascular compression syndromes: what the radiologist needs to know. Insights Imaging 2020;11:48. [Crossref] [PubMed]
- Merrett ND, Wilson RB, Cosman P, et al. Superior mesenteric artery syndrome: diagnosis and treatment strategies. J Gastrointest Surg 2009;13:287-92. [Crossref] [PubMed]
- Esmat HA, Najah DM. Superior mesenteric artery syndrome caused by acute weight loss in a 16-year-old polytrauma patient: A rare case report and review of the literature. Ann Med Surg (Lond) 2021;65:102284. [Crossref] [PubMed]
- Kirchhoff P, Geibel JP. Role of calcium and other trace elements in the gastrointestinal physiology. World J Gastroenterol 2006;12:3229-36. [Crossref] [PubMed]
- Tang L, Cheng CY, Sun X, et al. The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity. Front Physiol 2016;7:245. Erratum in: Front Physiol 2016;7:315. [PubMed]
- de Boer FJ, van Ieperen I, Boersma HE, et al. Severe hypocalcaemia and hypomagnesemia presenting with severe neurologic and gastro-intestinal symptoms: a case report and review of literature. CJEM 2021;23:401-3. [Crossref] [PubMed]
- Di Filippo L, Formenti AM, Rovere-Querini P, et al. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine 2020;68:475-8. [Crossref] [PubMed]
- Abobaker A, Alzwi A. The effect of COVID-19 on parathyroid glands. J Infect Public Health 2021;14:724-5. [Crossref] [PubMed]
Cite this article as: Selva Raj SR, Han GH, Karupiah M, Nagendram SV, Kang WH. COVID-19 infection with severe hypocalcaemia and superior mesenteric artery syndrome—a case report. AME Case Rep 2024;8:54.


