Use of fibrinolytics for percutaneous drainage of intrabdominal hematoma: a case report
Highlight box
Key findings
• Percutaneous drainage with concurrent intracavity administration of fibrinolytics has potential to be safe and effective choice for non-operative drainage of intrabdominal hematomas.
What is known and what is new?
• Intrabdominal hematoma has been historically treated conservatively with pain control and supportive management. In few cases, angioembolization, surgical drainage or percutaneous drainage may be employed for persistent hematomas that fail medical management. At the same time, intrapleural hematomas have been a point of interest for percutaneous drainage using fibrinolytics.
• Fibrinolytic administration for intrabdominal hematoma has not been widely studied in current literature. In this case, we administered tissue plasminogen activator (t-PA) and deoxyribonuclease (DNase) for an intrabdominal hematoma which was not amenable to drainage alone. Fibrinolytic administration helped us avoid surgical drainage in the patient.
What is the implication, and what should change now?
• Our case report shows the potential for concomitant administration of t-PA and DNase for resolution of intrabdominal hematoma. Fibrinolytic therapy did not result in any hematologic or coagulopathic complications. This case report serves as a way to instill further interest in large scope trials investigating indications, contraindications, safety and efficacy of this non-surgical therapeutic option.
Introduction
Traditionally, small intrabdominal hematomas are managed conservatively. In cases with expanding hematomas or ones associated with active bleeding, an interventional radiological approach or surgical correction may be required. In cases with symptomatic contained hematomas that do not display evidence of expansion, a percutaneous drainage may be employed. There is not a lot of literature on specific indications for management of intrabdominal hematomas. There has been, however, discussions on specific indications of rectus sheath hematoma (1). Based on our practice, percutaneous drainage has been employed for contained large intrabdominal hematomas associated with pain and/or pressure symptoms.
The use of fibrinolytics for effective percutaneous drainage of fluid collections has been a study of interest in the past. Intrapleural administration of fibrinolytics for infections has been extensively studied, but there is limited evidence of fibrinolytic therapy for intrabdominal fluid collections. Tissue plasminogen activator (t-PA)-deoxyribonuclease (DNase) therapy has been used increasingly for intrapleural fluid collections secondary to infections since the Second Multicenter Intrapleural Sepsis Trial (MIST2) (2). As for intrabdominal hematoma, the treatment choice is based on a variety of factors including, but not limited to symptoms, stability of hematoma, size of hematoma, and underlying pathology. Percutaneous drainage of intrabdominal hematoma has been used for large and stable hematomas, but surgical treatment has been employed for large, stable, and symptomatic hematomas that are not amenable to percutaneous drainage alone. In this report, we replicated the MIST2 fibrinolytic protocol for intrabdominal hematoma which was not amenable to drain placement alone. With this, we hope to instill further interest in future investigations into non-operative management of intrabdominal hematomas. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-178/rc).
Case presentation
A 51-year-old female with a history of multiple anti-reflux surgeries including a total gastrectomy with Roux-en-Y reconstruction, multiple hiatal hernia surgeries, revision of roux limb for candy cane syndrome, fascial dehiscence, and multiple jejunostomy tube (J-tube) placements presented to the clinic with complaint of postprandial abdominal discomfort and feeling of food getting stuck. She was found to have a large hiatal hernia containing transverse colon. She underwent laparotomy for hiatal hernia repair, intraoperative esophagogastroduodenoscopy, and J-tube replacement. Her course was complicated by acute blood loss anemia on postoperative day (POD) 2 with a hemoglobin of 6.9 g/dL. Computed tomography (CT) scan of the abdomen revealed a moderately sized intrabdominal hematoma. There was no evidence of active bleeding or increase in size of the hematoma. Hence, based on her stable hematoma and history of multiple abdominal surgeries with the most recent one being 2 days ago, decision was made to manage the hematoma conservatively with transfusion and pain control. She was eventually discharged on POD 13 on oral diet with J-tube feed supplementation.
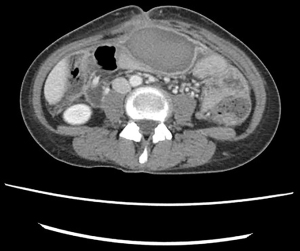
Unfortunately, she was readmitted on POD 26 with persistent severe abdominal pain and excessive leakage of succus around J-tube due to the pressure on the distal loop from the hematoma. She was afebrile with a normal white blood cell count of 9.7×109/L. CT scan revealed persistent hematoma that was completely walled off (Figure 1). Pain control was achieved with a regimen of oral (PO) acetaminophen, intravenous (IV) fentanyl, and IV ketorolac. She underwent an ultrasound-guided 14-Fr pigtail catheter placement that did not result in complete drainage of the hematoma and continued to have minimal output.
Although there was an increased concern for retained hematoma, we were reluctant to take her to the operating room (OR) based on her history of multiple abdominal surgeries. A decision was made to instill t-PA and DNase via the pigtail catheter into the hematoma. Two rounds of intrapleural protocol for intrapleural lytic (a total of 6 doses) were administered. Each dose consisted of 10 mg of t-PA and 5 mg of DNase. The drains were flushed daily with normal saline. Repeat CT scans showed significantly decreased size of hematoma (Figure 2). Her hemoglobin was repeated on the third day of starting lytic therapy which was stable at 11.2 g/dL. Her baseline hemoglobin was 11.8 g/dL. The patient reported clinical resolution of pain with no leakage around the J-tube. She was discharged on oral feeds after removal of J-tube and pigtail catheter. All events since index procedure are cited in the table based on events relating to POD (Table 1).
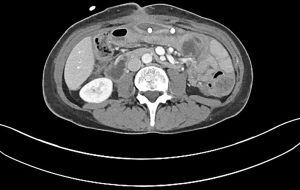
Table 1
| POD | Events |
|---|---|
| POD 0 | Open hiatal hernia repair, intraoperative esophagogastroduodenoscopy, and J-tube replacement |
| POD 2 | Finding of blood loss anemia with a CT abdomen finding concerning for intrabdominal hematoma |
| POD 2–13 | Conservative management and watchful monitoring of the intrabdominal hematoma |
| POD 13 | Discharge from the hospital |
| POD 26 | Readmission for persistent abdominal pain and excessive leakage around J-tube |
| POD 27 | Ultrasound-guided pigtail catheter was placed in the walled off persistent intrabdominal hematoma |
| POD 31–36 | Fibrinolytic therapy course |
| POD 39 | Discharge with resolved intrabdominal hematoma |
POD, postoperative day; J-tube, jejunostomy tube; CT, computed tomography.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The MIST2 was a large study undertaken in 2011 that showed evidence of improved drainage of infected fluid in patients with pleural infections when combined treatment with t-PA and DNase was given. The underlying pathophysiology of infected fluids comprises of fibrinous septations and presence of extracellular DNA along with infective components that increase the viscosity of the infected fluid, hence making simple percutaneous drainage difficult. DNase helps in cleaving the extracellular DNA while t-PA works as a fibrinolytic to remove the fibrinous septations which leads to removal of any loculations and drainage of infected fluid through a percutaneous drain. We applied the same rationale of breaking down fibrinous component of the retained intrabdominal hematoma in our patients. Apart from studies related to intrapleural fibrinolytic therapy, recent years have seen increased focus on multiple studies and trials evaluating the safety and efficacy of fibrinolytic evacuation of intracerebral and/or intraventricular hematomas (3-5).
However, use of fibrinolytic drugs in the management of retained intrabdominal hematoma has not been widely studied in current literature. We believe that a large multicenter study evaluating the safety and efficacy of this novel therapeutic approach for intrabdominal cases is long overdue. There have been a few studies and case reports studying the safety and effects of urokinase on percutaneous drainage of abdominopelvic hematoma or abscesses (6,7). However, urokinase has since been discontinued with better and more effective t-PA available as an alternative. The safety and efficacy profile of t-PA along with adjuvant action of DNase may yield superior results in patients with intrabdominal hematomas. This novel minimally invasive therapy can serve as an excellent non-operative treatment option for intrabdominal hematoma.
Studies done so far regarding intrabdominal fibrinolytic therapy do not show an increase in risk of hemorrhage, even in the setting of concurrent use of systemic anticoagulation (8). Lahorra et al. did not report bleeding complications or changes in physiologic coagulation parameters in their study about safety of urokinase use for percutaneous drainage of intracavitary abscesses (6). Similarly, Beland et al. did not report any bleeding complication with the use of t-PA for drainage of complex abdominal and pelvic abscesses (9). This finding was replicated in our patient who did not show any clinical signs of coagulopathy after fibrinolytic administration.
Our patient had a completely walled off hematoma that was radiologically evaluated with the help of CT scan of abdomen pelvis with IV contrast. This may have played a role in yielding successful results after intra-collection fibrinolytic administration. Further investigation regarding selection of patients who are good candidates for this approach should yield better and far more precise indications for this therapy.
The role of percutaneous drain placement should always be evaluated for risk vs. benefit profile in every clinical scenario. The benefits of drainage should outweigh the risks of placing percutaneous drain. The complications associated with percutaneous drainage described in the literature include infection, fistula formation, bleeding, sepsis, damage to surrounding structures and drain dislodgment (10). The benefits include secondary infection of the fluid collection, resolution of pain, and resolution of pressure symptoms caused by particularly large hematomas.
Conclusions
Patients with localized and contained hematomas may have well-developed clots, hence making percutaneous drainage difficult. We propose using the same protocol employed in MIST2 trials for evacuation of such hematoma. Our case showed significant reduction and eventual resolution in hematoma with the use of six doses of t-PA and DNase. Few studies have been performed about the use of fibrinolytics in treatment of intrabdominal hematomas. Even the ones which have been performed, urokinase was used in those studies. With improved fibrinolytics currently available, there is much study to be done for their safe use in intrabdominal hematoma collections and development of future protocols.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-178/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-178/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-178/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shikhman A, Tuma F. Abdominal Hematoma. In: StatPearls. Treasure Island: StatPearls Publishing; 2021.
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Dey M, Stadnik A, Awad IA. Thrombolytic evacuation of intracerebral and intraventricular hemorrhage. Curr Cardiol Rep 2012;14:754-60. [Crossref] [PubMed]
- Goulay R, Naveau M, Gaberel T, et al. Optimized tPA: A non-neurotoxic fibrinolytic agent for the drainage of intracerebral hemorrhages. J Cereb Blood Flow Metab 2018;38:1180-9. [Crossref] [PubMed]
- Hanley DF, Thompson RE, Muschelli J, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol 2016;15:1228-37. [Crossref] [PubMed]
- Lahorra JM, Haaga JR, Stellato T, et al. Safety of intracavitary urokinase with percutaneous abscess drainage. AJR Am J Roentgenol 1993;160:171-4. [Crossref] [PubMed]
- Vogelzang RL, Tobin RS, Burstein S, et al. Transcatheter intracavitary fibrinolysis of infected extravascular hematomas. AJR Am J Roentgenol 1987;148:378-80. [Crossref] [PubMed]
- Shenoy-Bhangle AS, Gervais DA. Use of fibrinolytics in abdominal and pleural collections. Semin Intervent Radiol 2012;29:264-9. [Crossref] [PubMed]
- Beland MD, Gervais DA, Levis DA, et al. Complex abdominal and pelvic abscesses: efficacy of adjunctive tissue-type plasminogen activator for drainage. Radiology 2008;247:567-73. [Crossref] [PubMed]
- De Filippo M, Puglisi S, D'Amuri F, et al. CT-guided percutaneous drainage of abdominopelvic collections: a pictorial essay. Radiol Med 2021;126:1561-70. [Crossref] [PubMed]
Cite this article as: Naqvi SJH, Voppuru SR, Wigle D. Use of fibrinolytics for percutaneous drainage of intrabdominal hematoma: a case report. AME Case Rep 2024;8:50.