
Sotorasib as first-line therapy in patients with advanced non-small cell lung cancer with KRAS gene mutations combined with brain metastases: a case report
Highlight box
Key findings
• Sotorasib, a new KRAS inhibitor, is currently recognized as the newest clinically targeted agent with apparent clinical efficacy in non-small cell lung cancer (NSCLC) patients with KRAS G12C mutations.
What is known and what is new?
• Sotorasib has been approved by the Food and Drug Administration (FDA) for marketing in the United States, and phase I/II clinical trials have been completed and phase III trials are under way, but the Chinese market has not been included in this clinical trial.
• The use of sotorasib in this patient provides a reference for the application of this clinical trial in China.
What is the implication, and what should change now?
• The report of this case provides a possibility for the treatment of NSCLC patients with KRAS G12C mutations, and sotorasib may become a first-line drug in the future.
Introduction
Non-small cell lung cancer (NSCLC) accounts for 80–85% of all lung cancers (1,2). The incidence of NSCLC is increasing yearly in China, with 5-year survival rates of 14–49% for stage I–IIIA of NSCLC and less than 5% for stage IIIB/IV (3,4). Nevertheless, many experts are dedicated to NSCLC research, and breakthroughs in its treatment have been achieved in recent years, significantly reducing the mortality rate (4,5). The progress in the treatment of malignant tumors is attributed mainly to improvements in the systemic treatment of advanced disease, including the approval of targeted therapies for patients with specific oncogenic mutations and the use of checkpoint inhibitors as monotherapy or in combination with chemotherapy for patients with apparent genetic mutations (6,7).
In NSCLC, KRAS G12C mutations occur in up to 14% of cases (8) and have been considered untargetable for many years, mainly due to the high toxicity and low specificity of the chemo regimens (9,10). Sotorasib (AMG-510) is a small molecule that binds explicitly inactive GDP-bound KRAS G12C, rendering it inactive and blocking oncogenic signaling. Sotorasib is a new KRAS inhibitor received by the U.S. Food and Drug Administration (FDA) in 2021 for KRAS-mutant NSCLC treatment approved for patients (10,11). However, many patients and experts in China are in a wait-and-see mode due to uncertainty about its safety, adverse effects, and accessibility (12-14). So, the needs of the vast population of patients in China are never to be satisfied completely. This case will provide some experience for scholars ranging from lung cancer to the oncology field. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-153/rc).
Case presentation
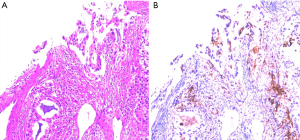
An 82-year-old male patient was admitted to the China-Japan Friendship Hospital in February 2022 mainly due to unresponsiveness with left-sided limb weakness after positron emission tomography-computed tomography (PET-CT) revealed an irregular soft tissue density mass with a size of about 6.1 cm × 5.9 cm visible in the upper lobe of the right lung of the chest and a slightly dense nodule with a size of about 1.5 cm visible in the right frontal lobe of the brain. He underwent computed tomography (CT)-guided lung occupancy under local anaesthesia. He underwent a biopsy under local anesthesia. The tissue was sent for pathology and genetic testing, which suggested adenocarcinoma of the lung with intermediate differentiation and a diagnosis of NSCLC with T3N0M1b (brain metastasis). He had a previous history of hypertension for more than 10 years, which was uncontrolled by medication, and no history of liver disease, hepatitis, or alcohol abuse. The patient underwent NGS testing covering 520 cancer-related genes to identify potentially actionable therapeutic targets. NGS analysis identified genetic alterations including KRAS G12C, NF1 C4110 + 2T, STK11 P281Afs, AR Y572C (Figure 1).
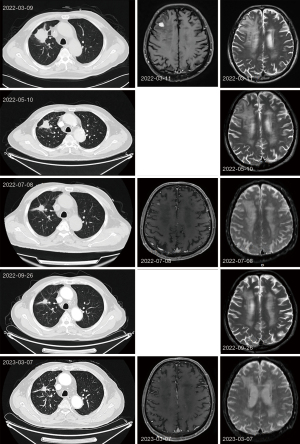
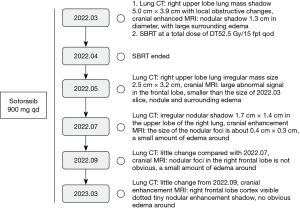
After the multidisciplinary team (MDT) evaluation, surgical treatment was not recommended, and then after multidisciplinary expert discussion, because of the presence of G12C mutation in KRAS and the foreign clinical data of sotorasib suggesting a disease control rate of 88.1% (15). Direct targeted therapy sotorasib could be considered as first-line treatment. The following treatment regimen was given: sotorasib 960 mg q.d. by oral, combined with stereotactic body radiation therapy (SBRT) for single intracranial metastases at a total dose of tumor (DT) 52.5 Gy/15 fpt q.o.d., and periodic tumor efficacy assessments were performed. The patient did not experience any adverse reactions or related safety events during the oral administration of the targeted medicine. The patient has been taking the drugs regularly and has not experienced any decrease in white blood cells, platelet, hemoglobin, and abnormal hepatic and renal function. The patient started taking sotorasib orally in March. Tumor-related examinations were performed about three months during the treatment period to observe the tumor progression, including imaging examinations [chest CT, cranial magnetic resonance imaging (MRI)] and laboratory tests. The tumor evaluation was partial remission (PR) on July 8, 2022. Imaging examinations [chest CT, cranial enhanced MRI and laboratory tests (routine blood, liver and kidney function, tumor markers)] were performed on March 7, 2023. The tumor was evaluated as stable disease (SD) (Figure 2). After more than one year of targeted therapy, the quality of life of the patient was significantly improved, sotorasib was used to control the patient’s tumor progression and significantly relieved the patient’s clinical symptoms, such as dyspnea, wheezing, dizziness, and nausea. At present, patients can perform a certain amount of physical exercise without affecting respiratory function (Figure 3).
All procedures performed in this study followed institutional or National Research Council ethical standards and the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient to publish this case report and accompanying images. A copy of the written consent form is available for review by the editorial board of this journal.
Discussion
Surgical resection is usually considered the primary modality of choice for treating lung tumors. However, many patients with advanced age factors may not be suitable for surgery after diagnosis. They have to rely on radiotherapy and chemotherapy. Given the higher incidence of NSCLC, its chemotherapy regimens have been evaluated inconsistently, and most patients have rapid tumor progression during chemo. With the advancement of scientific research and the development of genetic precision medicine, targeted therapy is receiving more attention from medical experts and scientists. In our case, the first-line treatment regimen for patients was sotorasib, which is currently in phase III clinical trials currently (15). The most common adverse events reported with sotorasib were pneumonia, hepatotoxicity, and diarrhea (9). In our case, after more than one year of targeted treatment with sotorasib 960 mg q.d. by oral, the patient did not show any significant abnormalities in liver function on regular review and did not complain of pneumonia or diarrhea-related symptoms during the drug administration. Considering the patient’s advanced age, surgery was not recommended. Sotorasib was used to control the patient’s tumor progression without affecting the patient’s quality of life and significantly relieved the patient’s clinical symptoms, such as dyspnea, wheezing, dizziness, and nausea.
Conclusions
Through this case, we can take regular oral sotorasib to control or even shrink the tumor extent in patients with advanced NSCLC without specific chronic diseases to obtain surgical opportunities to remove the lesions. Alternatively, long-term regular oral sotorasib can inhibit tumor progression for aged patients, prolonging the life cycle. Significantly, it reduces clinical discomfort and improves quality of life. So, this regimen can be provided as one of the choices for the particular population as the previous lines for the patients with KRAS G12C mutation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-153/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-153/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-153/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study followed institutional or National Research Council ethical standards and the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient to publish this case report and accompanying images. A copy of the written consent form is available for review by the editorial board of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Osmani L, Askin F, Gabrielson E, et al. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol 2018;52:103-9. [Crossref] [PubMed]
- Friedlaender A, Addeo A, Russo A, et al. Targeted Therapies in Early Stage NSCLC: Hype or Hope? Int J Mol Sci 2020;21:6329. [Crossref] [PubMed]
- Arbour KC, Riely GJ. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA 2019;322:764-74. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Kumar M, Sarkar A. Current therapeutic strategies and challenges in NSCLC treatment: a comprehensive review. Exp Oncol 2022;44:7-16. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Sankar K, Gadgeel SM, Qin A. Molecular therapeutic targets in non-small cell lung cancer. Expert Rev Anticancer Ther 2020;20:647-61. [Crossref] [PubMed]
- Hong DS, Fakih MG, Strickler JH, et al. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med 2020;383:1207-17. [Crossref] [PubMed]
- Nakajima EC, Drezner N, Li X, et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin Cancer Res 2022;28:1482-6. [Crossref] [PubMed]
- Koga T, Suda K, Fujino T, et al. KRAS Secondary Mutations That Confer Acquired Resistance to KRAS G12C Inhibitors, Sotorasib and Adagrasib, and Overcoming Strategies: Insights From In Vitro Experiments. J Thorac Oncol 2021;16:1321-32. [Crossref] [PubMed]
- Zhang SS, Nagasaka M. Spotlight on Sotorasib (AMG 510) for KRAS (G12C) Positive Non-Small Cell Lung Cancer. Lung Cancer (Auckl) 2021;12:115-22. [Crossref] [PubMed]
- Lee A. Sotorasib: A Review in KRAS G12C Mutation-Positive Non-small Cell Lung Cancer. Target Oncol 2022;17:727-33. [Crossref] [PubMed]
- Strohbehn GW, Sankar K, Qin A, et al. An evaluation of sotorasib for the treatment of patients with non-small cell lung cancer with KRASG12C mutations. Expert Opin Pharmacother 2022;23:1569-75. [Crossref] [PubMed]
- Soulières D, Gelmon KA. Sotorasib: Is Maximum Tolerated Dose Really the Issue at Hand? J Clin Oncol 2021;39:3427-9. [Crossref] [PubMed]
- de Langen AJ, Johnson ML, Mazieres J, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet 2023;401:733-46. [Crossref] [PubMed]
Cite this article as: Jia X, Liu M, Cheng Z. Sotorasib as first-line therapy in patients with advanced non-small cell lung cancer with KRAS gene mutations combined with brain metastases: a case report. AME Case Rep 2024;8:48.


