
Partial response to crizotinib + regorafenib + PD-1 inhibitor in a metastatic BRAF V600EMT colon cancer patient with acquired C-MET amplification and TPM4-ALK fusion: a case report
Highlight box
Key findings
• A rarely case of tropomyosin 4 (TPM4)-anaplastic lymphoma kinase (ALK) fusion co-occurring with mesenchymal-to-epithelial transition factor (MET) amplification in a colorectal cancer (CRC) patient with the Raf murine sarcoma viral oncogene homolog B (BRAF) V600E mutation who achieved partial response (PR) after the combined therapy of programmed cell death protein 1 (PD-1) inhibitor (tislelizumab), MET/ALK inhibitor (crizotinib) plus multikinase inhibitor (regorafenib), and whose response was monitored by continuous circulating tumor DNA (ctDNA) detection.
What is known and what is new?
• BRAF V600E mutation showed a poor outcome than those with non-V600E mutations.
• FDA approved doublet regimen for metastatic CRC (mCRC) patients with BRAF V600E mutation after first-line therapy, but not to mention the real application of ALK fusion inhibitor in CRC treatment.
• Liquid biopsy may help clinicians to tailor treatment and forecast patient prognosis, especial for tissue size may not be enough to conduct next-generation sequencing.
What is the implication, and what should change now?
• A novel combination of crizotinib, tislelizumab and regorafenib is a more effective and safer option for co-existence BRAF V600E, c-MET amplification and ALK fusion co-existence patient.
• CtDNA monitor is indispensable during the treatment of mCRC patients and can be served as a promising tool during their follow-up.
Introduction
Colorectal cancer (CRC) is among the most lethal and prevalent malignancies in the world and its incidence continues to rise among patients aged 40–49 (1). Advances in novel therapy development and scientific drug regimen design have significantly prolonged the survival of patients with CRC. However, these are far from meeting the urgent needs in clinics (2-4).
The Raf murine sarcoma viral oncogene homolog B (BRAF) mutation is detected in 8–10% of metastatic CRCs (mCRCs) and is strongly correlated with patients’ poor prognosis (5,6). It was reported that patients with BRAF V600E mutation showed a poor outcome than those with non-V600E mutations (7). Recently, studies on therapies for BRAF-mutated mCRC patients have obtained several advances. The BEACON subgroup analysis showed patients who received doublet (encorafenib plus cetuximab) therapies showed improved median overall survival (OS) compared with the patients treated with FOLFOXIRI regimen (fluorouracil, folic acid and irinotecan) (8). Based on this, the Food and Drug Administration (FDA) approved doublet regimen for mCRC patients with BRAF V600E mutation after first-line therapy (9).
Anaplastic lymphoma kinase (ALK) has been found fused to various genes in diverse cancers (10) and a variety of ALK fusions resulting in constitutive activation of ALK have been identified in human cancers (11). Mesenchymal-to-epithelial transition factor (MET) is the tyrosine kinase receptor for hepatocyte growth factor, and more recently, activating mutations and copy number amplification in MET have been recognized as potentially important therapeutic targets in non-small cell lung cancer (NSCLC) (12-14). However, both MET amplification and tropomyosin 4 (TPM4)-ALK fusion are of extremely lower frequencies (<2%) in mCRC, and few studies have described them as an acquired mutation (15,16). Not to mention the real application of their inhibitors in CRC treatment.
In this study, we identified a rarely reported TPM4-ALK fusion co-occurring with MET amplification in a CRC patient with BRAF V600E mutation who achieved partial response (PR) after the combined therapy of programmed cell death protein 1 (PD-1) inhibitor (tislelizumab), MET/ALK inhibitor (crizotinib) plus multikinase inhibitor (regorafenib), and whose response was monitored by continuous ctDNA detection. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-155/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
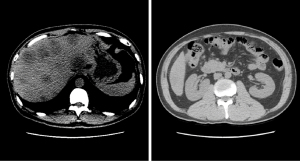
A 49-year-old Chinese male with right upper abdominal pain for more than 6 months was admitted to Beijing Hospital (Beijing, China) on October 10, 2020. Laboratory examination indicated the serum levels of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 125 (CA125) and alpha-fetoprotein was 46.3 ng/mL, >12,000 U/mL, 932.6 U/mL and 3 ng/mL, respectively. The colonoscopy showed a tumor (mass) located at the hepatic flexure. There was stenosis but no obstruction. The computed tomography (CT) and magnetic resonance imaging (MRI) results exhibited that there were more than 30 unresectable liver metastases (Figure 1). From the biopsy, the histopathological and molecular diagnosis suggested low-grade poorly differentiated adenocarcinoma with a BRAF V600E mutation but wild type kirsten rat sarcoma viral oncogene homologue (KRAS), neuroblastoma-RAS (NRAS), and phosphoinositide 3-kinase alpha (PIK3CA). The patient did not harbor erb-b2 receptor tyrosine kinase 2 (HER2) amplification, and PD-1/programmed cell death ligand 1 (PD-L1) staining showed negative. We finally diagnosed the patient as colon adenocarcinoma with liver metastases according to the National Comprehensive Cancer Network (NCCN) 2021 guideline for colon cancer (17). The tumors were unresectable with clinical risk score (CRS) of 4. The stage of the tumor was cT3N+?M1. The patient’s tissue and blood samples were subjected to next-generation sequencing (NGS), and the results showed the presence of BRAF V600E mutation with a mutant allele frequency of 27.92% (Figure 2). The abdominal pain exacerbated so rapidly within 10 days that the patient could not stand up, necessitating an urgent treatment.
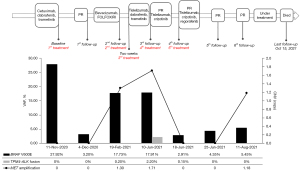
The patient received a series of treatments as shown in Figure 2. Considering the rapid progression of the tumor and worsening of liver function, he first received a combination therapy of triplet targeted agents: an epidermal growth factor receptor (EGFR) inhibitor, cetuximab (500 mg/m2), a BRAF inhibitor, dabrafenib (150 mg, BID), and a mitogen-activated protein kinase kinase 1 (MEK) inhibitor, trametinib (2 mg, QD) for six cycles. It achieved a satisfactory efficacy with a dramatic decrease of bilirubin, CEA, CA19-9 and CA125. On December 4, 2020, the patient’s blood sample was sent for NGS again, which showed that BRAF V600E mutation frequency was significantly reduced to 3.2% (Figure 2). Subsequent MRI examination showed that the patient achieved PR according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 (Figure 3).
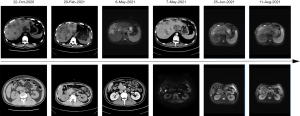
However, because the patient was not tolerant to this regimen and vomited a lot during the treatment, we changed the therapy into a vascular endothelial-derived growth factor (VEGF) inhibitor, bevacizumab combined with FOLFOXIRI (fluorouracil, folic acid and irinotecan) for one cycle. Unfortunately, his CA19-9, CA125, total bilirubin (TBIL) and direct bilirubin (DBIL) increased rapidly within two weeks (Tables 1,2). Liver MRI also showed that some nodules were larger than previous. It seemed the conventional triplet chemotherapy regimen did not work on this patient.
Table 1
| Biomarkers | Oct. 2020 | Nov. 2020 | Dec. 2020-1st | Dec. 2020-2nd | Jan. 2021-1st | Jan. 2021-2nd | Feb. 2021-1st | Feb. 2021-2nd | Feb. 2021-3rd | Mar. 2021 | Apr. 2021 | May 2021 | Jun. 2021 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CEA (ng/mL) | 46.3 | 136.1 | 27.8 | 11.7 | 3.8 | 2.8 | 3.8 | 7.1 | 9.4 | 11.2 | 10.7 | 16.9 | 17 |
| CA19-9 (U/mL) | 12,000 | 12,000 | 12,000 | 10,902 | 3,946 | 10,816 | 12,000 | >12,000 | >12,000 | >12,000 | 4,129 | >12,000 | 3,546.1 |
| CA125 (U/mL) | 932.6 | 1,713 | 488 | 227 | 178 | 331 | 659 | 1,361 | 1,320 | 577 | 104 | 134 | 95.4 |
CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125.
Table 2
| Biomarkers | Feb. 2021 | Mar. 2021 | Apr. 2021 | May 2021 |
|---|---|---|---|---|
| TBIL (μmol/L) | 81.6 | 39.2 | 23.4 | 18.9 |
| DBIL (μmol/L) | 61.2 | 27.3 | 13.2 | 8.3 |
| TBA (μmol/L) | 78.7 | 3.4 | 2.9 | 5.2 |
| GGT (U/L) | 1521 | 914 | 507 | 425 |
TBIL, total bilirubin; DBIL, direct bilirubin; TBA, total bile acid; GGT, glutamyltranspeptidase.
It has been reported that BRAF V600E CRC patients treated with the spartalizumab (PDR001), dabrafenib plus trametinib were well-tolerated and had favorable and durable response (18). After a thorough consultation within our medical team, communication with the patient and his family, the patient decided to receive this regimen. To avoid severe side effects, the patient was treated only with tislelizumab (200 mg, q3w) and trametinib (2 mg QD) first from March 1. The therapy was well-tolerated by this patient for 1 week, thus we added dabrafenib (150 mg, BID) for a better efficacy. During this period, TBIL decreased to 23.4 µmol/L and tumor biomarkers were generally stable (CEA and CA19-9 decreased and then increased, while CA125 continued to decrease) (Table 1). MRI and CT on May 6th showed that multiple metastases in the liver became smaller and some even disappeared, suggesting the treatment was effective (Figure 3). The regimen was then continued for about 3 months.
To further evaluate the efficacy, we performed NGS once again. Although the combined regimen of tislelizumab, trametinib and dabrafenib achieved good clinical efficacy, the BRAF V600E mutation frequency increased to 17.91%, accompanied by MET amplification (copy number ratio: 1.71) and TPM4-ALK fusion (frequency: 2.20%) (Figure 2).
Considering the presence of MET amplification and TPM4-ALK fusion, the patient finally agreed to change his regimen again and received a combination therapy of crizotinib (250 mg, QD) with tislelizumab (200 mg, Q3W) for 1 week. It was surprising that tumor biomarkers such as CA19-9 (from >12,000 to 3,546.1 U/mL) and CA125 (from 134 to 95.4 U/mL) decreased dramatically without any severe side effects (Table 1). One week later we added regorafenib (40 mg, QD). MRI scans on June 25 showed a PR in the primary tumor and liver metastases according to RECIST v1.1 (19) (Figure 2). NGS results showed BRAF V600E mutation frequency reduced to 4.35%, and previously co-occurring MET amplification and TPM4-ALK fusion were undetectable. This therapy has been continued up until now.
At the penultimate follow-up on August 11, abdominal MRI scan showed the local lesion and multiple liver metastases were slightly smaller. However, the wall of the flexura coli presented edema and became thicker than before. NGS analysis of peripheral blood showed a recurrence of the MET acquired resistant amplification mutation (Figure 2). It was assessed as PR (Figure 2). The patient is still under treatment of crizotinib, tislelizumab and regorafenib and within good physical condition. At the last follow-up on October 11, symptomatic treatment for obstructive jaundice continued to fail and the patient died on October 15, 2021. The patient finally achieved 11-month OS.
Discussion
Recently, with the emergence of liquid biopsy as a promising method for early diagnosis, therapeutic outcome assessment and prognosis prediction of tumor, plasmatic BRAF allele fraction (AF) has been demonstrated, and validated as an accurate prognostic factor in BRAF CRC treated with BRAF inhibitors (20,21). Circulating tumor DNA (ctDNA)-based dynamic monitoring has outstanding significance in reflecting the response of patients with advanced CRC. Currently, there are several studies on the prognostic value of ctDNA in CRC. Monitoring the change of ctDNA provided a comprehensive view of the patient’s tumor burden, and therefore helps to guide clinical decision-making throughout the disease course.
It is generally believed that BRAF mutations are mutually exclusive to RAS mutations, although co-existence has been reported in Beijing Hospital (22). The prevalence of BRAF mutations was recently reported to be as high as 21% in CRC patients in Norwegian registry (23) and 20.9% in Beijing Hospital (22). Patients with BRAF V600E mutation have been reported to have the worst prognosis among all BRAF mutations (7,24,25). Encouragingly, the development of novel targeted therapy has been proved to have fewer side effects and has a more promising efficacy (2). Previous studies have reported that dabrafenib plus trametinib was a new therapy with clinically meaningful anti-tumor activity and a manageable safety profile in patients with BRAF V600E NSCLC (26). Several studies have shown that RNF43-mutated represents a new biomarker for its potential to help prioritize anti-EGFR/BRAF combinations in mCRC BRAF V600E patients (27,28). However, this case is RNF43 wild type in both tissue and plasma NGS analysis. The patient also adopted this regimen combined with a PD-1 inhibitor, tislelizumab. It did work on him and had acceptable short-time response.
During the treatment, we detected the c-MET amplification and TPM4-ALK fusion by NGS after triplet targeted therapy, which suggesting that the usage of their inhibitor would further improve the efficacy. Before this case, only one patient with BRAF-mutated and MET amplification had been reported, and this patient had benefited from conversion from anti-EGFR and -BRAF inhibition to a MET inhibitor plus BRAF inhibitor-induced tumor response (29). Since there was no safety or efficacy data, we just chose the low dose of crizotinib and regorafenib combined with tislelizumab. This therapy achieved a good clinical therapeutic effect without severe treatment-related side effects on this patient, thus providing a novel regimen design for CRC patients with c-MET amplification or TPM4-ALK fusion. This case uncovered liquid biopsy may help clinicians to tailor treatment and forecast patient prognosis, especial for tissue size may not be enough to conduct NGS. This case also demonstrated the importance of continuous monitoring of ctDNA during treatment, which could adjust or change the regimen based on the detection results for a better application of precision medicine.
Conclusions
In conclusion, we reported a rare case of a mCRC patient who presents co-existence of a BRAF V600E mutation, c-MET amplification and TPM4-ALK fusion. We continuously monitored the sequencing results of ctDNA, and changed the therapy regimen according to the results during the treatment, which showed that the novel combination of crizotinib, tislelizumab and regorafenib is a more effective and safer option for this type of patients. The frequency of driver gene mutations varied with the alteration of different regimen. Meanwhile, it would also result in whether the patient responded to the targeted therapy or progressed. The patient eventually achieved 11-month OS. Taken together, ctDNA monitor is indispensable during the treatment of mCRC patients and can be served as a promising tool during their follow-up.
Acknowledgments
We would like to thank the patient and his family for providing consent to participate in this study, as well as all research staff and co-investigators involved.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-155/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-155/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-155/coif). Z.L. is an employee of Geneplus-Beijing (Beijing, China). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Musetti C, Garau M, Alonso R, et al. Colorectal Cancer in Young and Older Adults in Uruguay: Changes in Recent Incidence and Mortality Trends. Int J Environ Res Public Health 2021;18:8232. [Crossref] [PubMed]
- Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther 2020;5:22. [Crossref] [PubMed]
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306-15. [Crossref] [PubMed]
- Rimassa L, Bozzarelli S, Pietrantonio F, et al. Phase II Study of Tivantinib and Cetuximab in Patients With KRAS Wild-type Metastatic Colorectal Cancer With Acquired Resistance to EGFR Inhibitors and Emergence of MET Overexpression: Lesson Learned for Future Trials With EGFR/MET Dual Inhibition. Clin Colorectal Cancer 2019;18:125-132.e2. [Crossref] [PubMed]
- Popovici V, Budinska E, Tejpar S, et al. Identification of a poor-prognosis BRAF-mutant-like population of patients with colon cancer. J Clin Oncol 2012;30:1288-95. [Crossref] [PubMed]
- Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer 2015;51:587-94. [Crossref] [PubMed]
- Guan WL, Qiu MZ, He CY, et al. Clinicopathologic Features and Prognosis of BRAF Mutated Colorectal Cancer Patients. Front Oncol 2020;10:563407. [Crossref] [PubMed]
- Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol 2021;39:273-84. [Crossref] [PubMed]
- Array BioPharma Inc. Braftovi® (encorafenib) capsules. Prescribing information; 2020.
- Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer 2013;13:685-700. [Crossref] [PubMed]
- Grande E, Bolós MV, Arriola E. Targeting oncogenic ALK: a promising strategy for cancer treatment. Mol Cancer Ther 2011;10:569-79. [Crossref] [PubMed]
- Jenkins RW, Oxnard GR, Elkin S, et al. Response to Crizotinib in a Patient With Lung Adenocarcinoma Harboring a MET Splice Site Mutation. Clin Lung Cancer 2015;16:e101-4. [Crossref] [PubMed]
- Waqar SN, Morgensztern D, Sehn J. MET Mutation Associated with Responsiveness to Crizotinib. J Thorac Oncol 2015;10:e29-31. [Crossref] [PubMed]
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850-9. [Crossref] [PubMed]
- Oddo D, Siravegna G, Gloghini A, et al. Emergence of MET hyper-amplification at progression to MET and BRAF inhibition in colorectal cancer. Br J Cancer 2017;117:347-52. [Crossref] [PubMed]
- Raghav K, Morris V, Tang C, et al. MET amplification in metastatic colorectal cancer: an acquired response to EGFR inhibition, not a de novo phenomenon. Oncotarget 2016;7:54627-31. [Crossref] [PubMed]
- Members NCCP. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)-Colon Cancer. 2021 V2. NCCN; 2021.
- Corcoran R, Giannakis M, Allen J, et al. SO-26 Clinical efficacy of combined BRAF, MEK, and PD-1 inhibition in BRAFV600E colorectal cancer patients. Ann Oncol 2020;31:S226-7. [Crossref]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Tie J, Cohen JD, Wang Y, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut 2019;68:663-71. [Crossref] [PubMed]
- Ros J, Matito J, Villacampa G, et al. Plasmatic BRAF-V600E allele fraction as a prognostic factor in metastatic colorectal cancer treated with BRAF combinatorial treatments. Ann Oncol 2023;34:543-52. [Crossref] [PubMed]
- Huang Y, Jia W, Zhao G, et al. Analysis of driver gene mutations in colorectal cancer by using next-generation sequencing. Chinese Journal of Geriatrics 2021:646-9.
- Taieb J, Lapeyre-Prost A, Laurent Puig P, et al. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br J Cancer 2019;121:434-42. [Crossref] [PubMed]
- Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004;116:855-67. [Crossref] [PubMed]
- Dankner M, Rose AAN, Rajkumar S, et al. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene 2018;37:3183-99. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJM, et al. Phase 2 Study of Dabrafenib Plus Trametinib in Patients With BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. J Thorac Oncol 2022;17:103-15. [Crossref] [PubMed]
- Quintanilha JCF, Graf RP, Oxnard GR. BRAF V600E and RNF43 Co-mutations Predict Patient Outcomes With Targeted Therapies in Real-World Cases of Colorectal Cancer. Oncologist 2023;28:e171-4. [Crossref] [PubMed]
- Elez E, Ros J, Fernández J, et al. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAF(V600E) metastatic colorectal cancer. Nat Med 2022;28:2162-70. [Crossref] [PubMed]
- Ros J, Elez E. Overcoming acquired MET amplification after encorafenib-cetuximab in BRAF-V600E mutated colorectal cancer. Eur J Cancer 2022;172:326-8. [Crossref] [PubMed]
Cite this article as: Huang Y, Zhang S, Hu X, Wang X, Zhao Y, Li Z. Partial response to crizotinib + regorafenib + PD-1 inhibitor in a metastatic BRAF V600EMT colon cancer patient with acquired C-MET amplification and TPM4-ALK fusion: a case report. AME Case Rep 2024;8:38.


