
The coexistence of papillary thyroid carcinoma, anaplastic thyroid carcinoma (squamous cell carcinoma subtype) and poorly differentiated thyroid carcinoma: a case report
Highlight box
Key findings
• We report a rare case of the coexistence of papillary thyroid carcinoma (PTC), anaplastic thyroid carcinoma-squamous cell carcinoma (ATC-SCC) subtype and poorly differentiated thyroid carcinoma (PDTC).
What is known and what is new?
• The simultaneous occurrence of PTC, ATC-SCC subtype, and PDTC is extremely rare in clinical terms or literature reports. The treatment has not been standardized, and early radical surgery is the first choice.
• This manuscript adds relevant guidelines for the current treatment: the current treatment principle for coexisting tumor is combination therapy to target each tumor component.
What is the implication, and what should change now?
• Since ATC-SCC and PDTC are highly invasive, clinicians should pay close attention to this disease, and they should be diagnosed and treated as early as possible. Thorough surgical resection is the key element to improve prognosis, and postoperative follow-up is also particularly important. In addition, the combination of adjuvant therapies such as thyroid-stimulating hormone (TSH) suppressive therapy, radiotherapy, chemotherapy and 131I may further improve the prognosis of the patient.
Introduction
Papillary thyroid carcinoma (PTC) is the most common thyroid cancer. Most primary squamous cell carcinoma of thyroid (PSCCT) patients have a favorable prognosis with a 10-year survival rate of more than 90% (1). PSCCT is a primary malignant epithelial tumor of the thyroid consisting entirely of differentiated squamous epithelial cells, with an incidence of less than 1% of thyroid malignancies (2). The latest classification of endocrine and neuroendocrine tumors in the 5th edition of World Health Organization (WHO) has classified PSCCT as a subtype of anaplastic thyroid carcinoma (ATC). As a subtype of ATC, the clinical manifestations and biological behavior of PSCCT are similar to ATC, which is highly invasive, and the age of onset is often over 50 years old. The majority of ATC patients are already in advanced stages at initial diagnosis and usually have a poor prognosis (3). Poorly differentiated thyroid carcinoma (PDTC) is a kind of thyroid carcinoma showing follicular cell differentiation, systematic and biological behavior between differentiated carcinoma (PTC and follicular carcinoma) and undifferentiated carcinoma (anaplastic carcinoma), which is rare clinically, accounting for about 3–5% of all thyroid cancers (4,5). The presence of a rapidly growing mass in the neck is the most common symptom and sign of PDTC. The proportion of distant metastasis in PDTC patients is as high as 37–85% (6), which tends to invade the recurrent laryngeal nerve, trachea or esophagus, and clinical symptoms such as hoarseness, dyspnea and dysphagia may occur at the late stage of the disease.
Thyroid tumor with the above three pathological types at the simultaneous occurrence is extremely rare in clinical terms or literature reports (7), the diagnosis and treatment of such multicomponent tumors is currently very challenging. Here, we report a rare case of the coexistence of PTC, ATC-squamous cell carcinoma (SCC) subtype and PDTC. The clinical course, radiographic and histopathological diagnosis, and treatment of current literature are present. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-192/rc).
Case presentation
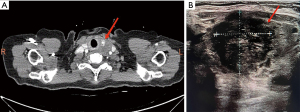
A 69-year-old female patient was admitted to Department of Oncology Surgery of Qinghai University Affiliated Hospital with a chief complaint for presenting with a history of left neck mass for one month. No complaints of pain in the neck mass, dysphagia and dyspnea, and she denied a history of radiation to the neck or family history of thyroid carcinoma. Physical examination showed a mass approximately measuring 2 cm × 1 cm on the left thyroid gland on palpation, which was firm with an irregular surface, moved up and down during deglutition. No palpable cervical lymphadenopathy was present. Initial laboratory tests, including thyroid function tests, were unremarkable. The thyroid ultrasound showed a hypoechoic nodule with unclear boundary, labeled in Thyroid Imaging Reporting and Data System (TI-RADS) as four, measuring 16 mm × 8 mm in the left thyroid gland (Figure 1A). Thyroid computed tomography (CT) revealed a space-occupying lesion in the left lobar of the thyroid gland, with malignant nature being considered, and compression of the internal jugular vein in the left side (Figure 1B). An ultrasound-guided fine-needle aspiration biopsy (FNAB) of the left thyroid gland revealed a large number of patchy follicular epithelial cells and atypical changes were seen in a few cells, it showed that a definitive diagnosis would require further intraoperative freezing. The reason why FNAB is not enough to detect malignant tumors may be that tumor cells exist firmly in the tumor through the reaction of promoting connective tissue hyperplasia and fibrosis, resulting in unsuccessful puncture; or the sampling error caused by less acupuncture tissue due to the uneven distribution of tumor cells in the tissue.
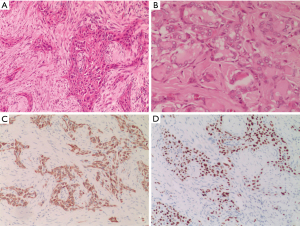
Surgical treatment was performed after perfecting the preoperative preparation. Intraoperatively, we observed a grayish-white mass approximately measuring 2 cm × 1.5 cm on the left thyroid gland, which was firm with unclear borders, invaded the thyroid peritoneum and the strap muscle. The boundary between the mass as well as trachea and esophagus was not clear. Then the left thyroid mass was removed radically and intraoperative freezing showed, malignant. Finally total thyroidectomy with radical left cervical lymph node dissection was performed. One lymph node metastasis was found in the resected lymph node tissue. The postoperative pathology was diagnosed as PTC with squamous cell differentiation, considering SCC, and some areas of poorly differentiated carcinoma. Microscope observation (Figure 2A,2B): papillary carcinoma and SCC with invasive growth. Papillary carcinoma cells were glandular tubular and papillary with large nuclei and hairy glass-like. While squamous carcinoma cells were irregularly distributed in strips and clusters with long spindle-shaped, or irregular polygonal cells, large and deeply stained nuclei, eosinophilic cytoplasm, obvious nuclear atypia, and easy to see nuclear mitotic figure as well as intracellular keratinisation and inter-cellular bridges. Immunohistochemically: tumor cells were positive for CK19 (Figure 2C), P63 (Figure 2D), CK5/6 (+), AE1/AE3 and Galectin-3, and negative for thyroid transcription factor-1 (TTF-1), Tg, CD56, calcitonin (CT), presynaptic (Syn) and CgA, the proliferative index was approximately 30%. Polymerase chain reaction (PCR) showed BRAFV600E gene mutation. Among them, CK5/6, CK19 and P63 expression were positive, CT, CgA and SYN were negative, which were consistent with the immunohistochemical characteristics of PTC and PDTC. AE1/AE3, CK5/6, CK19, p63 were all positive, while TTF-1 and Tg expression were negative. Combined with pathological morphology, this case was supported be of the ATC-SCC subtype.
Calcium supplementation and fluid replacement treatment were given to the patient after operation. Four weeks after the surgery, 131I treatment (100mCi) was given. Then thyroid-stimulating hormone (TSH) suppressive therapy were used until now. Paclitaxel (300 mg d1–2) combined with cisplatin (30 mg d1–4) chemotherapy was performed 50 days after the surgery, and three cycles were completed (21 days as a cycle). At the same time, the whole neck radiotherapy including the range of lymph nodes in Ib, II, III, IV, V and VI regions of the neck was performed in the third cycle of chemotherapy. The planned target radiotherapy dose was 150 Gy/25 F. During the post-operative period of 5 months, on follow-up, no obvious abnormalities were seen in the patient.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Timeline
During the 1st–9th days after hospitalization, laboratory examination and preoperative evaluation were performed. The 10th day was operation day. The 11th–13th days were postoperative duration. The duration from 4 weeks after operation to 5 months is follow-up period (Figure 3).
Discussion
The occurrence of SCC in the thyroid gland is uncommon, because the normal thyroid gland has no squamous epithelial cells (8). Once SCC has been detected from fine needle aspiration results or pathological specimens of thyroid gland, primary SCC needs to be differentiated from secondary SCC according to their diverse clinical and biological behaviors. In order to make the precise treatment planning and improve the patient’s prognosis, it is necessary to determine whether the patient has metastatic SCC of nasopharynx, larynx, trachea, esophagus or lungs to the thyroid gland (8). So, we performed the head, neck and chest computed tomography, gastrointestinal angiography, laryngoscope, gastrointestinal endoscopy and other auxiliary examinations, excluded the primary lesions of adjacent and distant SCC. PSCCT, as a subtype of ATC, has many similarities in clinical manifestation and pathological histopathology with ATC. The cell subtypes of ATC mainly include sarcoma-like cells (spindle cells), epithelial cells (squamous cells), pleomorphic giant cells, and variety of cell mixtures (9,10). In addition to histopathology, the diagnosis of ATC-SCC subtype also depends on immunohistochemistry (9). AE1/AE3 expression in ATC is often positive, while Tg and TTF-1 are often negatively expressed. Previous studies have reported that positivity of CK5/6, CK19, P63 and p40 are helpful for making the final diagnosis of PSCCT (10). In this case, postoperative pathology suggested squamous cell differentiation, and SCC was considered. In addition, immunohistochemistry showed that AE1/AE3, CK5/6, CK19, p63 expression were all positive, while that of TTF-1 and Tg expression were negative, which supported that this case was of the ATC-SCC subtype.
Currently, the etiology of ATC-SCC subtype is not yet clear, so the tumor origin is relatively confused. Three hypotheses about the etiology are as follows (11): (I) originating from embryonic remnants of the remnant branchial arch or the thyroglossal duct, (II) a few researchers deduced that it is derived from complicated metaplasia of chronic thyroid diseases, (III) de-differentiation of existing anaplastic, papillary, or medullary carcinomas. In the present case, we observed coexistence of classic papillary carcinoma with SCC. Histopathology showed intermingled and transitional zones of typical papillary carcinoma and SCC. This suggests that squamous metaplasia arises from the follicular epithelium and facilitates the development of SCC. Therefore, we opt for the “de-differentiation theory”.
More surprisingly, there were two other kinds of histological components, PTC and PDTC, in the pathological tissue of this patient. The histological structure of PTC showed infiltrative growth, often with follicles, which were flat and rounded, and the appearance of sand bodies was a characteristic sign of papillary carcinoma with diverse cell morphology, pseudo inclusion bodies in the nucleus, fine nucleoli, etc. In 2022, the 5th edition of the WHO identified PDTC as an independent entity subtype of thyroid tumors, which histological features are mainly solid, trabecular and island growth patterns, accompanied by obvious necrosis and vascular invasion. The tumor cells have uniform morphology, small cells, chromatin staining, unclear nucleolus, and mitotic figures are common (12). The histopathology of patient showed that the tumor cells were distributed in solid flakes and nests, small follicular structures were seen between the cell nests, the interstitial fibrous blood vessels were unclear, and the tumor often invaded the capsule. Some areas were poorly differentiated cancer, accompanied by obvious necrosis, and mitotic figures were seen, at the same time, squamous cell was observed in some tumor tissues. Among the immunohistochemical markers of PDTC, Tg, TTF-1, Galectin3, CK5/6 and p53 were often positively expressed, CK19 expression was partly positive, CT, CgA and Syn expression were often negative. In a few cases, CgA and Syn were weakly positive (12). In our case, the tumor cells were positive for CK5/6, CK19 and P63, and they were negative for CT, CgA and Syn, which were the evidences to help diagnose PDTC. Therefore, according to the evidence of histopathology, immunohistochemistry and auxiliary examinations, a case of coexistence of PTC, ATC-SCC subtype and PDTC was finally diagnosed.
The pathological tissue of the patient showed the coexistence of three kinds of tumor components, which is extremely rare in clinical practice and literature reports, making it a therapeutic challenge. The current treatment principle for coexisting tumor is combination therapy to target each tumor component (13). According to the American Joint Committee on Cancer (AJCC) 8th edition tumor node metastasis (TNM) staging of thyroid cancer and the 2021 American Thyroid Association (ATA) updated Guidelines For The Management Of Patients With Undifferentiated Thyroid Cancer, the patient’s clinical stage was pT3bN1M0, stage IVB. The guidelines recommend treatment options including: radical surgery, 131I therapy, systemic chemotherapy and neck radiotherapy. Previous studies have reported that surgery combined with 131I therapy is the main treatment of PDTC, and total thyroidectomy with lymph node dissection is the first choice for PDTC. The 131I-avidity was found in 70–80% of PDTC patients, providing the basis for 131I therapy. It is suggested that 131I therapy should be considered in PDTC patients after operation (14). Furthermore, external beam radiation therapy (EBRT) has been recognized as a necessary choice for patients with PDTC or ATC to reduce local recurrence and improve prognosis. Sanders et al. have shown that EBRT should be considered for diseases with tumor size ≥4 cm, extrathyroid invasion, lymph node metastasis or inoperable radical surgery (15).
As a subtype of ATC, PSCCT may refer to the National Comprehensive Cancer Network (NCCN) guidelines (version 2022) for ATC treatment options, which suggests that in addition to 131I and ERBT, paclitaxel combined with carboplatin or other chemotherapy regimens can be used for surgically resectable ATC. However, an Updates on the Management of Thyroid Cancer reported that ATC-SCC subtype was less sensitive to chemotherapy and had radiation resistance (16), making it difficult to improve the survival benefit of ATC patients by radiotherapy and chemotherapy. For the moment, the importance of surgical treatment of ATC is still emphasized, but such tumors are highly malignant. They are often accompanied by invasion of trachea, esophagus, recurrent laryngeal nerve, anterior cervical muscle group and posterior tissue at the time of diagnosis, which makes it so difficult to obtain radical resection. Previously related case reports showed that for patients who failed to achieve R0 resection with lymph node metastasis, the average survival time was only 6–12 months despite applying adjuvant therapy such as radiotherapy and chemotherapy (17). Therefore, thorough surgical resection is a key element to effectively improve the prognosis (18), and the patients should then be closely monitored after surgery to detect recurrence as soon as possible. In addition, it has been reported that targeting programmed cell death protein 1/programmed death ligand-1 (PD-1/PD-L1) and TP53 is beneficial to the prognosis of ATC-SCC. Some studies have shown that BRAFV600E is the most common gene mutation in ATC (19), and the ATA guidelines has recommended molecular targeted therapy (dabrafenib/trimetazidine) for unresectable locally advanced ATC lesions, which may improve the progression-free survival rate of ATC patients (20). The BRAFV600E mutation of this patient was positive, and the targeted drug dabrafenib could be applied. However, the efficacy of targeted therapy and immunotherapy still remains uncertain due to the lack of a strong evidence-based rationale.
Conclusions
In conclusion, we reported an extremely rare case of coexistence of three tumor tissues, which is challenging in diagnosis and treatment. Usual imaging examinations cannot make a firm diagnosis, and the diagnosis was mainly based on postoperative histopathology and immunohistochemistry. Since ATC-SCC and PDTC are highly invasive, they should be diagnosed and treated as early as possible. Thorough surgical resection is the key element to improve prognosis, and postoperative follow-up is also particularly important. In addition, 131I therapy, EBRT and chemotherapy are the main adjuvant therapies for such thyroid tumors, and whether emerging therapies based on molecular targeting and immunization are effective deserves further study.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-192/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-192/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-192/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deng Y, Li H, Wang M, et al. Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw Open 2020;3:e208759. [Crossref] [PubMed]
- Shakib H, Rajabi S, Dehghan MH, et al. Epithelial-to-mesenchymal transition in thyroid cancer: a comprehensive review. Endocrine 2019;66:435-55. [Crossref] [PubMed]
- Syed MI, Stewart M, Syed S, et al. Squamous cell carcinoma of the thyroid gland: primary or secondary disease? J Laryngol Otol 2011;125:3-9. [Crossref] [PubMed]
- Xu B, Ghossein R. Poorly differentiated thyroid carcinoma. Semin Diagn Pathol 2020;37:243-7. [Crossref] [PubMed]
- Sassolas G, Hafdi-Nejjari Z, Remontet L, et al. Thyroid cancer: is the incidence rise abating? Eur J Endocrinol 2009;160:71-9. [Crossref] [PubMed]
- de la Fouchardière C, Decaussin-Petrucci M, Berthiller J, et al. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer 2018;92:40-7. [Crossref] [PubMed]
- Dong S, Song XS, Chen G, et al. Mixed primary squamous cell carcinoma, follicular carcinoma, and micropapillary carcinoma of the thyroid gland: A case report. Auris Nasus Larynx 2016;43:455-9. [Crossref] [PubMed]
- Jang JY, Kwon KW, Kim SW, et al. Primary squamous cell carcinoma of thyroid gland with local recurrence: ultrasonographic and computed tomographic findings. Ultrasonography 2014;33:143-8. [Crossref] [PubMed]
- Lui JT, Khalil MN, Chandarana SP. Primary squamous cell of the thyroid-an abbreviated clinical presentation. J Otolaryngol Head Neck Surg 2014;43:17. [Crossref] [PubMed]
- Lam AK. Squamous cell carcinoma of thyroid: a unique type of cancer in World Health Organization Classification. Endocr Relat Cancer 2020;27:R177-92. [Crossref] [PubMed]
- Othman RT, Baizeed AMA, Mohammed AA. Squamous cell carcinoma of the thyroid gland in an elderly female presenting as a rapidly enlarging thyroid mass. Int J Surg Case Rep 2020;70:119-22. [Crossref] [PubMed]
- Bongiovanni M, Bloom L, Krane JF, et al. Cytomorphologic features of poorly differentiated thyroid carcinoma: a multi-institutional analysis of 40 cases. Cancer 2009;117:185-94. [PubMed]
- Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 2016;126:1052-66. [Crossref] [PubMed]
- Walvekar RR, Kane SV, D'Cruz AK. Collision tumor of the thyroid: follicular variant of papillary carcinoma and squamous carcinoma. World J Surg Oncol 2006;4:65. [Crossref] [PubMed]
- Sanders EM Jr. An evidence-based review of poorly differentiated thyroid cancer. World J Surg 2007;31:934-45. [Crossref] [PubMed]
- Araque KA, Gubbi S, Klubo-Gwiezdzinska J. Updates on the Management of Thyroid Cancer. Horm Metab Res 2020;52:562-77. [Crossref] [PubMed]
- Chu MMH, Mirza O, Bishop PW, et al. Primary squamous cell carcinoma of the thyroid gland successfully treated with surgical resection and adjuvant chemoradiotherapy. BMJ Case Rep 2021;14:e241209. [Crossref] [PubMed]
- Asioli S, Erickson LA, Righi A, et al. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol 2010;23:1269-78. [Crossref] [PubMed]
- Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012;22:1104-39. [Crossref] [PubMed]
- Cabanillas ME, Habra MA. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat Rev 2016;42:47-55. [Crossref] [PubMed]
Cite this article as: Zhang Y, Liu Q, Ma D, Maimaiti Y, Ma Z. The coexistence of papillary thyroid carcinoma, anaplastic thyroid carcinoma (squamous cell carcinoma subtype) and poorly differentiated thyroid carcinoma: a case report. AME Case Rep 2024;8:47.



