
Recurrent epithelioid angiomyolipoma of the adrenal gland: a case report and literature review
Highlight box
Key findings
• This is the first case report of recurrent adrenal epithelioid angiomyolipoma (EAML).
What is known and what is new?
• EAML, a subtype of angiomyolipoma, is distinct. It has a biologic behavior of borderline tumor, a malignant tendency, and a risk of metastasis and recurrence. Adrenal EAML is very rare.
• A case about recurrent adrenal EAML is offered. We describe the symptoms at the onset, what imageological examination we did and how to treat.
What is the implication, and what should change now?
• Adrenal EAML has a risk of recurrence. It is important to probability of the recurrence and related risk factors.
Introduction
PEComa, also known as a perivascular epithelioid cell tumor, is a rare group of tumors that originate from mesenchyme and exhibit a similar expression of melanocytes and myogenic markers (1). Vascular tissue, muscular cells, and adipose tissue make up angiomyolipoma (AML), which belongs to the PEComa family. Epithelioid angiomyolipoma (EAML) is a subtype of AML that is distinguished by its more aggressive growth and lower clinical outcomes. EAML is formed by cells that are organized into nests, sheets, or broad alveoli that are surrounded by vascular septa (2). PEComa has a borderline biological behaviour (3). More rare for EAML of adrenal origin. It is true that only six cases of adrenal EAML have been documented in the English-language literature. Long-term postoperative follow-up and recurrence cases are rarely reported. This article presents the clinical data of a case involving the recurrence of EAML in the adrenal gland. It is worth noting that this is the first documented report of such a case in the existing literature. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-189/rc).
Case presentation
The patient, a 60-year-old male, was admitted to the cardiology department due to chest tightness for 8 days. There were no other symptoms, no remarkable signs on physical examination, with previous history of hypertension. No abnormality was found on electrocardiogram, there were markers of myocardial injury. Blood chemistry analysis did not show any significant abnormalities. The enhanced computed tomography (CT) scan showed a circular mixed-density shade in the left retroperitoneum. The size is about 66×54 mm, with a mean CT number of about 46 HU. Within it there are speckled dense shadow, curved fat-density shadow and scattered slightly low-density shadow. The patient was transferred to urological surgical department and underwent a laparoscopic left adrenalectomy in July, 2017. The mass was surrounded by abundant blood vessels and adherence with surround-tissue.
Postoperative pathology: (left) adrenal epithelioid vascular smooth muscle lipoma, tumour size 7×6×2.5 cm, intact envelope, focal necrosis, extensive haemorrhage with calcium deposits, tumour cells with moderate atypia, mitotic count 1–3/10 high power field (HPF). There were no atypical mitoses of the tumor cells. Immunohistochemistry (IHC): (focal weak +), Ki-67 (1% +), HMB45 (weak +); Inhibin-a (−), CK (−), CgA (−), Syn (−), S-100 (−), CD56 Melan-A (−), Desmin (−). Microscopically, the tumour cells are polygonal, epithelioid, plasma-rich, hyaline and eosinophilic, with heterogeneous nuclei and nucleoli; they are arranged in a solid lamellar and glandular vesicular pattern. Mature adipose tissue and thick-walled blood vessels are seen focally. There is focal lymphocytic infiltration and pigmentation.
Regular annual review of the urological ultrasound showed no significant bilateral adrenal abnormalities.
In July 2022, the elderly male came to the emergency room with a day-old history of pain in his upper left abdomen and lumbar region. Prior medical history included hypertension and diabetes. On admission, no significant abnormalities were noted. Gastroscopy was completed and no significant abnormality was found. The enhanced CT scan of the abdomen showed: hypodense foci in the operated area were larger than before, and the surrounding exudate was more than the previous film; and multiple soft tissue nodules and masses in and around the left adrenal gland was markedly enhanced. No significant abnormalities in laboratory tests.
A left laparoscopic adrenalectomy was performed under general anesthesia in July 2022. Intraoperatively, two grey sphere-like masses of approximately 1 cm in size were found in the upper part of the left kidney. The patient was discharged 10 days later with symptomatic treatment with low molecular heparin. The patient did not complain of abdominal discomfort after discharge from the hospital.
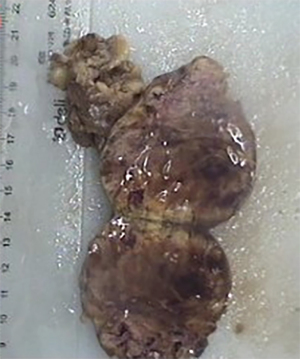
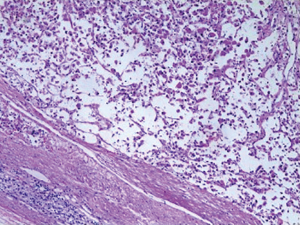
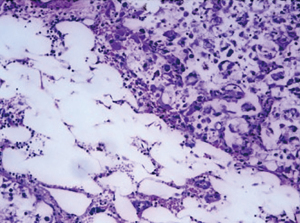
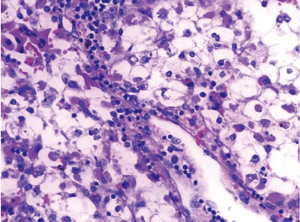
Postoperative pathology: left adrenal mass: two greyish grey-brown nodules, maximum diameter 0.9 to 1.1 cm, greyish grey-red-gray-brown in section, solid, medium quality (Figure 1), fully removed. Intact envelope (Figure 2), consistent with epithelioid vascular smooth muscle lipoma (epithelioid PEComa) (Figures 3,4), Some adipocyte cells and individual smooth muscle cells (Figure 5). There were hyperchromatic nuclei with prominent nucleoli with some mitotic figure and capsular invasion (Figures 6,7), IHC: CD34 (vascular +), Vimentin (partial +), Melan-A (+); CK (−), TFE-3 (−), D2-40 (−), Ki-67 (~3%), P53 (−), Inhibin-a (−), CD56 (−), SF-1 (−), HMB45 (−), SMA (−), Desmin (−), S-100 (−), Pax2 (−), Pax-8 (−). Special staining: reticulocyte silver stain (showing reticulocytes surrounding individual cells).

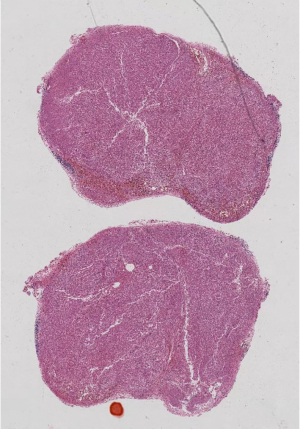
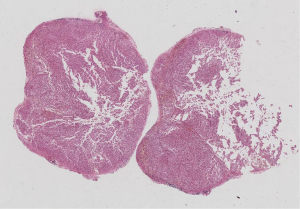
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PEComa is a mesenchymal tumor that is composed of perivascular epithelioid cells, according to the World Health Organization’s definition in 2002. The composition of PEComas consists of epithelioid cells and spindle cells, either one or both cells are stacked or nested. Significant native vessels are visible, and tumor cells are often arranged radially around the vessels. Among different lesions of the PEComa family, AML, and lymphangioleiomyomatosis are relatively common and are associated with tuberous sclerosis (4,5).
EAML is a unique subtype of AML. EAML has a set of characteristics that set it apart from classic AML. EAMLs are usually larger than AMLs, with epithelioid cells being the predominant component, but they often do not have the characteristic adipose tissue component of this type of neoplasm. Determining the malignant behavior, relapsing, and metastasis rates of EAMLs is not a straightforward task (6). Folpe et al. classified PEComas into three groups based on a few criteria, with benign PEComas, uncertain malignant potential (there are either nuclear pleomorphism or multinucleated giant cells, or cells that can reach a size of more than 5 cm), and malignant PEComas [meet two or more following criteria, including (I) the size of tumor is larger than 5 cm, (II) tumour cell infiltrating, (III) nuclear grade and cellularity of a high standard, (IV) a mitotic rate that exceeds one mitosis per 50 high-power fields, (V) tumor cell necrosis, (VI) vascular wall penetration] (7).
The prevalence of adrenal EAML is very low. We reviewed the literature that was produced from January 1, 1990 to September 1, 2022. We identified six full-text articles of six cases and carefully read and analyzed them. Details were given about the patient’s gender, age, tumor size, affected side, presentation, imaging characteristics, surgery, and follow-up results, as well as the first author and publication year (see Table 1).
Table 1
| Case No. | Author/year | Sex/age (years) | Size (cm) | Side | Presentation | Imaging | CT | Surgery | Follow-up (years) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-enhanced | Contrast-enhanced | |||||||||
| 1 | D’Antonio (8)/2009 | M/42 | 6.0×4.5 (CT) | L | Lank and back pain | CT/MRI | Miscellaneous | Heterogeneous contrast enhancement | L adrenalectomy | 1 |
| 2 | Lau (9)/2012 | F/55 | 11.0 (on specimen) | L | R-side thorax pain | CT | – | – | L adrenalectomy and nephrectomy | 3.6 |
| 3 | Komarowska (2)/2015 | M/35 | 16×9×10 (on specimen) | R | Abdominal pain | CT | – | – | R adrenalectomy and nephrectomy | 0.5 |
| 4 | Valeshabad (10)/2019 | F/33 | 7.3×6.9 (CT) | L | Abdominal discomfort | CT | – | Enhancing soft-tissue density mass | L adrenalectomy and nephrectomy | – |
| 5 | Torres Luna (11)/2020 | M/32 | 16×17×20 (CT) | R | R-sided lower back pain | CT/MRI | – | – | R adrenalectomy and nephrectomy | – |
| 6 | Cicek (12)/2022 | F/64 | 9.5×6.8 (CT) | L | Incidental finding | CT/US | – | Heterogeneous with smooth borders | L adrenalectomy | 1.5 |
| 7 | Present case/2017 | M/60 | 7.0×6.0×2.5 (on specimen) | L | Incidental finding | CT | – | Enhanced mass | L adrenalectomy | 5 |
| 8 | Recurrent case/2022 | M/65 | 1.1 (on specimen) | L | L abdominal and flank pain | CT | Miscellaneous | Markedly enhanced mass | L adrenalectomy | 0.17 |
CT, computerized tomography; M, male; L, left; MRI, magnetic resonance imaging; F, female; R, right; US, ultrasound.
According to the seven cases reported (including our present case and excluding our recurrent case), adrenal EAML patients were found to be between 32 and 64 years old and had a median age of 42 years. The age of onset tends to be younger, with 85.7% being young to middle-aged patients under the age of 60. Of the cases, five were on the left side, and two were on the right side. Notably, three of the seven cases were female, and of these patients, all (3/3) were on left side. Four of the seven cases were male, and of these patients, half (2/4) were on the left side and another half were on the right side. These patients showed different clinical symptoms, they were no clinical symptoms or abdominal, flank, or back pain. Nonfunctional adrenal EAMLs were present in all cases and had tumor sizes that ranged from 6.0–20.0 cm. Two of the cases developed distant metastases, to the lungs and liver respectively. The patient with pulmonary metastases died 43 months after diagnosis. Two cases were combined with tuberous sclerosis complex (TSC). All cases underwent adrenalectomy and, with the exception of this paper, no cases were followed up for more than 5 years after surgery.
The main treatment for EAML is radical resection. Chemotherapy and arterial embolization of the tumor are options for alternative treatment. Mammalian target of rapamycin (mTOR) inhibitors has also been tried (13).
Conclusions
Adrenal EAML does not have typical clinical symptoms. About 27% of patients with EAML have a history of TSC (1). The age of onset tended to be younger. The tumors were large in size at the time of discovery, with diameters greater than 6 cm, and their malignant tendency should be of greater concern. The final diagnosis of EAML is dependent on the immunohistochemistry of the pathology. Immunohistochemical staining is commonly positive for markers related to skin cancer like SOX10, HMB-45, HMB-50, Melan-A, MITF, NKIC3, and myogenic markers such as SMA, but not for markers related to the epithelium like cytokeratin (14). Adrenal EAML has a biologic behavior of borderline tumor with malignant potential and a risk of distant metastasis and recurrence. There is a pathological similarity between primary and recurrent adrenal EAML. Therefore, radical surgical resection should be considered as its necessary treatment regardless of its being the primary or recurrent tumor. Long-term postoperative follow-up is an important part of the treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-189/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-189/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-189/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Breda A, Ogunyemi O, Leppert JT, et al. Flexible ureteroscopy and laser lithotripsy for single intrarenal stones 2 cm or greater--is this the new frontier? J Urol 2008;179:981-4. [Crossref] [PubMed]
- Komarowska H, Bednarek-Rajewska K, Kański M, et al. Epithelioid angiomyolipoma mimicking adrenal cortical carcinoma: A diagnostic pitfall. Oncol Lett 2015;10:2130-4. [Crossref] [PubMed]
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. [Crossref] [PubMed]
- Zarineh A, Silverman JF. Adrenal perivascular epithelioid cell tumor: a case report with discussion of differential diagnoses. Arch Pathol Lab Med 2011;135:499-502. [Crossref] [PubMed]
- Xuan LL, Wei JG, Liu HG. Pathological diagnosis and new progress of perivascular epithelioid cell tumor. Zhonghua Bing Li Xue Za Zhi 2021;50:282-7. [PubMed]
- Mete O, van der Kwast TH. Epithelioid angiomyolipoma: a morphologically distinct variant that mimics a variety of intra-abdominal neoplasms. Arch Pathol Lab Med 2011;135:665-70. [Crossref] [PubMed]
- Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 2005;29:1558-75. [Crossref] [PubMed]
- D’Antonio A, Caleo A, Caleo O, et al. Monotypic epithelioid angiomyolipoma of the adrenal gland: an unusual site for a rare extrarenal tumor. Ann Diagn Pathol 2009;13:347-50. [Crossref] [PubMed]
- Lau SK. Malignant PEComa of the adrenal gland. Pathol Res Pract 2012;208:113-7. [Crossref] [PubMed]
- Kord Valeshabad A, Kravis B, Bremer W, et al. Angiomyolipoma of the Adrenal Glands. Clin Genitourin Cancer 2019;17:e553-5. [Crossref] [PubMed]
- Torres Luna N, Mosquera JE, Comba IY, et al. A Primary Adrenal Epithelioid Angiomyolipoma (PEComa) in a Patient with Tuberous Sclerosis Complex: Report of a Case and Review of the Literature. Case Rep Med 2020;2020:5131736. [Crossref] [PubMed]
- Cicek M, Kazan HO, Atis RG, et al. Primary Epithelioid Angiomyolipoma of Adrenal Gland: Case Report and Literature Review. Prague Med Rep 2022;123:199-205. [Crossref] [PubMed]
- Wyluda E, Baquero G, Lamparella N, et al. Fatal malignant metastastic epithelioid angiomyolipoma presenting in a young woman: case report and review of the literature. Rare Tumors 2013;5:e46. [Crossref] [PubMed]
- Yang JW, Liang C, Yang L. Advancements in the diagnosis and treatment of renal epithelioid angiomyolipoma: A narrative review. Kaohsiung J Med Sci 2022;38:925-32. [Crossref] [PubMed]
Cite this article as: Lin Z, Ding Z, Jiang H. Recurrent epithelioid angiomyolipoma of the adrenal gland: a case report and literature review. AME Case Rep 2024;8:57.


