
A rare case report of mucinous adenocarcinoma exacerbated by long-standing solitary rectal ulcer syndrome
Highlight box
Key findings
• This case report illustrated the rare occurrence of rapid progression from solitary rectal ulcer syndrome (SRUS) to mucinous adenocarcinoma in a 29-year-old male patient.
• Surgical resection, combined with postoperative adjuvant FOLFOX chemotherapy, effectively controlled cancer progression.
• Immunohistochemical analysis revealed positive expression of MLH1(+), MSH2(+), MSH6(+), PMS2(+), and HER2(+), providing insights into the molecular characteristics of SRUS-associated mucinous adenocarcinoma.
What is known and what is new?
• SRUS is a chronic and uncommon rectal lesion with the potential for malignant transformation.
• This case adds to existing knowledge by presenting a rare instance of SRUS progressing to mucinous adenocarcinoma, highlighting the importance of early detection and management of SRUS-associated malignancies.
What is the implication, and what should change now?
• Clinicians should heighten awareness of the potential for cancerous transformation in SRUS patients, emphasizing regular monitoring and timely intervention.
• Early diagnosis and prompt treatment, including surgical resection and adjuvant chemotherapy, are crucial for improving outcomes in SRUS-associated malignancies.
• Further research is needed to elucidate underlying mechanisms and risk factors, guiding future clinical practice and treatment strategies.
Introduction
Solitary rectal ulcer syndrome (SRUS) is an unusual and long-lasting disorder of defecation with a symptom profile characterized by rectal bleeding, diarrhea, constipation, and anorectal pain, marked by recurrent episodes of remission and relapse (1). The incidence rate of SRUS is estimated at 1/100,000 per year (2). The endoscopic manifestations of SRUS are mostly ulcers, especially solitary ulcers, while the remaining cases range from hyperemic mucosa to broad-based polypoid formations with lesions of varying shapes and sizes (3). SRUS diagnosis highly relies on precise endoscopic examination and pathological evaluation. It is typically classified as a chronic benign inflammatory disorder with a higher incidence in young adult populations. However, there have been some case reports that suggested a potential for malignant transformation (4). In addition, mucinous adenocarcinoma from SURS is a rare manifestation of the disorder, and there is a paucity of information available on its diagnostic criteria, treatment modalities, and clinical outcomes. Hence, we present a case report of mucinous adenocarcinoma associated with SRUS. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-207/rc).
Case presentation
A 29-year-old male patient had suffered recurrent constipation and anal discomfort since 2013, without mucous or bloody stool. The patient denied any related significant past medical history or relevant family history. The local hospital had found rectal ulcers through colonoscopies, but the diagnosis of SRUS was not confirmed due to limited pathological diagnostic ability. Mesalazine suppository treatment was found to be ineffective for the patient based on the recommendations of local physicians. To further evaluate and treat the disease, he visited a hospital in Beijing in 2018. Pelvic magnetic resonance imaging (MRI) revealed a circumferential dense shadow in the rectal area and irregularity of the local intestinal wall, which was highly suspicious as fistula. The pathological examination results, as depicted in Figure 1, showed the presence of inflammation exudate, granulomatous formation, with local mucous formation observed. The immunohistochemical analysis revealed a patchy expression of p53(+) and an approximate 15% expression of Ki-67(+). Thus, SRUS was confirmed, based on the combination of clinical symptoms, colonoscopy, and pathological examination. A long-term administration of mesalazine enema was performed on the patient to relieve the symptoms. Between July 2019 and October 2020, the patient experienced recurrent episodes of fever accompanied by unexplained pain in the right buttock region. The administration of cephalosporin antibiotics resulted in temporary alleviation of the symptoms but prone to recur. In October 2020, the patient returned to that hospital again for further assessment and subsequent treatment. MRI revealed multiple defects in the middle and upper rectal walls, accompanied by the formation of perirectal abscesses. The fistula orifice on the posterior wall of the rectum had enlarged. And multiple lymph nodes were detected in the rectum, sigmoid mesentery, and both pelvic walls, with some showing enlargement. During colonoscopy, a substantial ulcerative lesion was identified in the rectum. The observed lesion was large, deep, and concave, measuring approximately 3 cm × 5 cm in length and occupying approximately 1/3 to 1/2 of the annular cavity depth in the rectum, located 6 cm proximal to the anal verge. In December 2020, due to the worsening of the patient’s discomfort symptoms, including fever and right buttock pain, it became necessary to perform a transverse colostomy. The discomfort symptoms were relieved after the transverse colostomy. Regrettably, 4 months post-surgery, the patient experienced a recurrence of these symptoms, accompanied by significant impairments in lower limb mobility, which were effectively mitigated through anti-infective therapy.

For further assessment and treatment, the patient was admitted to our hospital. After admission physical examination and evaluation, it was found that the patient had tenderness in his right hip. And a palpable mass was found 6 cm away from the dentate line on the posterior wall of the lower rectum, which was smooth on the surface, hard in texture, poorly mobile, and the upper edge of the mass was untouchable. The finger cot was stained with dark red blood. The anal sphincter contraction was normal. No obvious abnormality was found in the external genitalia. The patient still had recurrent fever after admission, with a maximum body temperature of 38.5 centigrade, accompanied with right hip pain. Blood tests showed white blood cell (WBC) 10.38×109/L, neutrophil (NEU) 8.15×109/L, high-sensitivity C-reactive protein (hs-CRP) 63.25 mg/L, carcinoembryonic antigen (CEA): 1.79 ng/mL, carbohydrate antigen 125 (CA125): 9.7 U/mL, CA199: 2.1 U/mL. The results of tuberculosis-specific antigen stimulation cytokine release test, G test, and rheumatoid factor test were all negative. Erythrocyte sedimentation rate (ESR) was 20 mm/h. The test of human immunodeficiency virus (HIV) antigen antibody, syphilis TP-ab, hepatitis B and C antigens were also negative. The fecal occult blood test was negative. Importantly, stool gene testing (SDC2 methylation) was performed twice for this patient and revealed a positive result for both times (8.27 and 9.3), indicating a higher risk of colorectal cancer. Colonoscopy revealed a submucosal mass located 6 cm from anus, with nodular elevation on the surface and covered with a large amount of purulent secretion, which could not be completely visualized after flushing. A fistula orifice was visible at the edge of the mass (Figure 2). Pelvic MRI showed an irregular mass at presacral location, measuring 44 mm × 75 mm × 107 mm. T2-weighted imaging (T2WI) and fat-saturation T2WI showed high signal, and a large number of septate-like low signals were visible inside the lesion, which involved the posterior wall of the rectum, bilateral piriformis muscles, right lavatorial muscle, and formed a fistula shadow in the right sciatic anal fossa. The MRI diagnosis suggested a mucinous adenocarcinoma in the pre-sacrum and behind the rectum, with a predominant component of mucus (Figure 3). Considering the patient’s recurrent symptoms and unclear diagnosis, a complicated-case-discussion was held at the hospital. This discussion suggested a high suspicion of presacral tumor caused by SRUS, and the possibility of malignancy could not be excluded as the disease progressed. Currently, internal medicine conservative treatment is ineffective, while surgery is indicated. On September 6, 2021, the patient underwent a surgical procedure under general anesthesia with endotracheal intubation, including sacral mass resection, pelvic floor reconstruction, partial rectal resection (distal closure), and bilateral ureteral stent implantation. The surgical approach involved a midline abdominal incision approximately 15 cm in length. Intraoperatively, exploration revealed a large, hard mass adjacent to the sacrum, adherent to the posterior wall of the mid-segment of the rectum. Dissection was carefully performed to avoid injury to adjacent structures, including the ureters and reproductive vessels. The rectum was dissected and resected, approximately 10 cm was excised, leaving approximately 4 cm of residual rectum from the anal verge. Rapid frozen section pathology confirmed mucinous adenocarcinoma intraoperatively, prompting distal rectal closure and drainage tube placement (Figure 4). The surgery proceeded smoothly with minimal blood loss (approximately 100 mL) and stable vital signs under satisfactory anesthesia. Postoperative pathology revealed mucinous adenocarcinoma in the sacral mass with negative resection margins, absence of vascular thrombi, and no lymph node metastasis. Immunohistochemistry showed positivity for MLH1, MSH2, MSH6, PMS2, and HER2, while Braf was negative. CD3 and CD8 staining demonstrated few scattered T lymphocytes in the tumor stroma, and Ki-67 proliferation index was approximately 35% positive in tumor cells. The concrete timeline of the treatment was shown in Table 1.
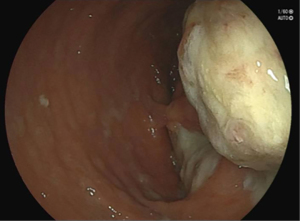
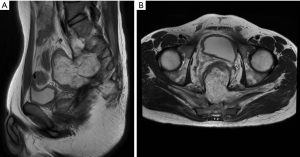
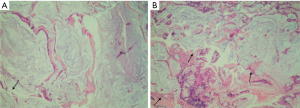
Table 1
| Time | Symptoms | Diagnosis | Examination | Treatment |
|---|---|---|---|---|
| 2013–2018 | Recurrent constipation and anal discomfort | SRUS | MRI; colonoscopy; pathological examination | Administration of mesalazine enema |
| July 2019–October 2020 | Fever; pain in the right buttocks | SRUS | – | Cephalosporin antibiotics |
| October 2020–December 2020 | Fever and right buttock pain | SRUS | MRI; colonoscopy | – |
| December 2020 | Worse fever and right buttock pain | SRUS | Preoperative systemic evaluation and cancer screening | Transverse colostomy |
| 2021 | Fever; right buttock pain; severe limitations in lower limb movement | SRUS: mucous adenocarcinoma | MRI; colonoscopy; pathological examination | Surgery resection; adjuvant chemotherapy |
SRUS, solitary rectal ulcer syndrome; MRI, magnetic resonance imaging.
Furthermore, the patient underwent genetic testing, and the chromosome copy map is provided as supplementary material (Figure S1). The results indicated a tumor mutational burden (TMB) value of 4.91 mut/Mb, microsatellite instability (MSI) of 0.13%, and copy number variation (CNV) of 0.02%, ranking among the top 23.5% of mucinous adenocarcinoma patients. Additionally, the PD-L1 immunohistochemical test yielded negative results. Approximately 1-month post-surgery, the patient received eight cycles of FOLFOX6 adjuvant chemotherapy, which included oxaliplatin, leucovorin, and fluorouracil, along with three traditional anti-colorectal cancer drugs. Pelvic radiotherapy commenced on September 17th, 2021, utilizing intensity-modulated radiation therapy (IMRT) with doses of gross tumor volume (GTV) 55 Gy/25 f and clinical target volume (CTV) 45 Gy/25 f. No notable discomfort was observed during the radiotherapy sessions. Postoperative MRI scans revealed partial rectal absence and a normal sigmoid stump (Figure S2). The patient had a smooth recovery without any significant complications over the subsequent 2-year period. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In this study, we presented a case of rapid progression from SRUS to mucinous adenocarcinoma within a short timeframe. The diagnosis was primarily established through colonoscopy examination and subsequent pathological assessment. Furthermore, immunohistochemical analysis of the pathological tissue demonstrated positive expression of MLH1(+), MSH2(+), MSH6(+), PMS2(+), and HER2(+). Surgical resection, combined with postoperative adjuvant FOLFOX chemotherapy, proved to be effective in managing the progression of SRUS-related mucinous adenocarcinoma during a 2-year follow-up period. In clinical practice, SRUS is a rare condition that is frequently misdiagnosed due to its ambiguous macroscopic appearance and the limited familiarity of clinicians with its histological characteristics. While most cases of SRUS are benign and exhibit a gradual progression, presenting symptoms may include rectal bleeding, diarrhea, constipation, and anorectal pain. However, rare occurrences of malignant transformation have been reported.
Unfortunately, despite the existence of several proposed hypotheses, the precise mechanism underlying the development of SRUS remains elusive. It is widely recognized that SRUS arises from repeated mucosal trauma, coupled with ischemia of the rectal wall resulting from excessive straining during defecation. Furthermore, occult or overt rectal prolapse and paradoxical contraction of the pelvic muscles have been associated with the development of SRUS, leading to ischemia and ulceration. The mechanisms underlying the transformation of SRUS into cancerous lesions are even less well understood. Mucinous adenocarcinoma represents a well-differentiated and rare histological subtype. It has been demonstrated that the risk of colorectal cancer in patients with ulcerative colitis (UC), which is also a type of ulcerative diseases, increases approximately 8–10 years after initial diagnosis (5). Chronic inflammation and heightened epithelial cell turnover in patients with UC contribute to the development of cellular dysplasia, which is closely linked to the development of colorectal cancer. Additionally, the progression of colorectal cancer in UC patients is associated with the generation of reactive oxygen species (ROS), activation of the Wnt/β-catenin pathway, and disruption of the intestinal microbiome (6). However, limited studies have sporadically reported on the transformation of SRUS into adenocarcinoma, with a relatively low incidence rate (3,7-11). A notable study, conducted at a single center in Pakistan from 1990 to 2011, reported on 116 cases of SRUS, revealing a mere 1.72% incidence of colonic adenocarcinomas, with only two cases identified (7).
Recent studies have identified two notable characteristics of mucinous adenocarcinoma associated with SRUS, fibromuscular obliteration and distorted glandular architectures (3,8). Multiple studies have demonstrated that up to 38% of patients with SRUS exhibit symptoms similar to those of sessile serrated polyps, suggesting a distinct pathway in the development of colorectal carcinogenesis (11). All of these studies, including ours, demonstrated the importance and urgency of developing accurate methods for early detection of SRUS canceration.
The immunohistochemical test results revealed positive expression of MLH1(+), MSH2(+), MSH6(+), PMS2(+), and HER2(+), all of which are associated with the mismatch repair (MMR) system. In clinical practice, tumors lacking expression of MLH1, MSH2, PMS2, or MSH6 are classified as deficient MMR (dMMR), while tumors expressing MLH1, MSH2, PMS2, or MSH6 are classified as proficient MMR (pMMR) (12). The MLH1, MSH2, MSH6, and PMS2 genes encode proteins that play a critical role in DNA MMR (13). Given the tumor heterogeneity and individual variations observed among patients, these genes hold reference significance in differentiating between SRUS canceration and malignant mucinous adenocarcinoma. Alterations in chromosome copy number can provide the tumor with the necessary material for adaptation and evolution.
There have been reports indicating that patients with mucinous colonic adenocarcinoma have a poorer prognosis compared to those with non-mucinous colonic adenocarcinoma (14,15). Nevertheless, the optimal treatment approach for mucinous adenocarcinoma in patients with SRUS remains uncertain. Currently, surgical resection, radiotherapy, and chemotherapy are the primary therapeutic modalities employed for the management of colorectal adenocarcinoma (16). In our case report, the patient underwent presacral tumor resection and pelvic partial rectal resection, followed by FOLFOX6 chemotherapy. FOLFOX6 has shown efficacy as an alternative regimen for the initial treatment of metastatic colorectal cancer (17). The modified FOLFOX-6 regimen has been regarded commonly as the adjuvant chemotherapy, after the colorectal tumor resection surgery (18). After a 2-year clinical follow-up, the patient exhibited a favorable prognosis, with no signs of local recurrence or distant metastasis. This case highlights the effectiveness of combining surgery with FOLFOX6 chemotherapy in controlling the progression of SRUS-related mucinous adenocarcinoma, providing valuable guidance for clinical diagnosis and treatment.
Conclusions
In conclusion, this case underscores the reliability of colonoscopy with pathological confirmation as a dependable diagnostic approach for SRUS and its cancerous transformation. Early-stage SRUS-related mucinous adenocarcinoma can be effectively managed through a combination of surgical resection and FOLFOX6 chemotherapy, resulting in improved patient outcomes and survival. It is anticipated that this research will enhance the understanding of SRUS and provide a clinical framework for the diagnosis and treatment of SRUS-associated cancerous conditions.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-207/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-207/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-207/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gouriou C, Siproudhis L, Chambaz M, et al. Solitary rectal ulcer syndrome in 102 patients: Do different phenotypes make sense? Dig Liver Dis 2021;53:190-5. [Crossref] [PubMed]
- Zhu QC, Shen RR, Qin HL, et al. Solitary rectal ulcer syndrome: clinical features, pathophysiology, diagnosis and treatment strategies. World J Gastroenterol 2014;20:738-44. [Crossref] [PubMed]
- Chiang JM, Changchien CR, Chen JR. Solitary rectal ulcer syndrome: an endoscopic and histological presentation and literature review. Int J Colorectal Dis 2006;21:348-56. [Crossref] [PubMed]
- D’Hoore A. Rectal prolapse, intussusception, solitary rectal ulcer. In: Herold A, Lehur PA, Matzel K, et al. editors. Coloproctology. Berlin, Heidelberg: Springer; 2017:135-46.
- Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol 2014;20:16389-97. [Crossref] [PubMed]
- Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett 2014;345:235-41. [Crossref] [PubMed]
- Abid S, Khawaja A, Bhimani SA, et al. The clinical, endoscopic and histological spectrum of the solitary rectal ulcer syndrome: a single-center experience of 116 cases. BMC Gastroenterol 2012;12:72. [Crossref] [PubMed]
- Tsuchida K, Okayama N, Miyata M, et al. Solitary rectal ulcer syndrome accompanied by submucosal invasive carcinoma. Am J Gastroenterol 1998;93:2235-8. [Crossref] [PubMed]
- Lambin T, Lafeuille P, Rivory J, et al. Adenocarcinoma arising from a long-standing solitary rectal ulcer syndrome. Endoscopy 2022;54:E205-6. [Crossref] [PubMed]
- Li SC, Hamilton SR. Malignant tumors in the rectum simulating solitary rectal ulcer syndrome in endoscopic biopsy specimens. Am J Surg Pathol 1998;22:106-12. [Crossref] [PubMed]
- Ball CG, Dupre MP, Falck V, et al. Sessile serrated polyp mimicry in patients with solitary rectal ulcer syndrome: is there evidence of preneoplastic change? Arch Pathol Lab Med 2005;129:1037-40. [Crossref] [PubMed]
- Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res 1998;4:1-6. [PubMed]
- Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 2006;7:335-46. [Crossref] [PubMed]
- Ott C, Gerken M, Hirsch D, et al. Advanced Mucinous Colorectal Cancer: Epidemiology, Prognosis and Efficacy of Chemotherapeutic Treatment. Digestion 2018;98:143-52. [Crossref] [PubMed]
- Maisano R, Azzarello D, Maisano M, et al. Mucinous histology of colon cancer predicts poor outcomes with FOLFOX regimen in metastatic colon cancer. J Chemother 2012;24:212-6. [Crossref] [PubMed]
- Luo C, Cen S, Ding G, et al. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond) 2019;39:13. [Crossref] [PubMed]
- Ducreux M, Bennouna J, Hebbar M, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer 2011;128:682-90. [Crossref] [PubMed]
- Nguyen TQ, Bui TO, Tran PT, et al. Modified Folfox6 as Adjuvant Chemotherapy in Vietnamese Patients With Colorectal Cancer. Cancer Control 2019;26:1073274819864111. [Crossref] [PubMed]
Cite this article as: Tan Q, Zhou J, Zhao K, Lian S, Li J, Huang Y, Qiu C, He J, Liu C. A rare case report of mucinous adenocarcinoma exacerbated by long-standing solitary rectal ulcer syndrome. AME Case Rep 2024;8:63.


