
Association between serum albumin and body water using a bioelectrical impedance analyzer: a case report of longitudinal variation in a child with initial idiopathic nephrotic syndrome
Highlight box
Key findings
• Longitudinal variations in serum albumin (S-Alb) levels and the extracellular water (ECW)/total body water (TBW) ratio in pediatric patients with initial idiopathic nephrotic syndrome (INS) were assessed using a body composition analyzer.
What is known and what is new?
• Although pediatric initial INS requires meticulous management, frequent blood tests are stressful for children.
• Longitudinal S-Alb levels and the ECW/TBW ratio showed a significantly strong negative correlation (r=−0.72, P<0.04).
What is the implication, and what should change now?
• The use of simple and reproducible body composition analysis could reduce the invasiveness of testing in children with INS.
Introduction
Body composition analyzers can potentially noninvasively assess serum albumin (S-Alb) levels. Bioelectrical impedance analysis (BIA) can predict body composition based on changes in electrical conductivity due to tissue composition. This is due to the relationship between resistance (R) and capacitive reactance (XC), BIA enables the determination of disease, nutrition, and moisture status using electrical characteristics (1). This mechanism enables the measurement of extracellular water (ECW), intracellular water (ICW), and total body water (TBW) (2). ECW can be used to compare the volume of excess water accumulating in the extracellular spaces in individuals of different sizes (3). BIA is characterized by being simple, inexpensive, and repeatable compared to conventional methods like deuterium oxide and sodium bromide, BIA is non-invasive and quick, making it useful for routine practice (4). The ECW/TBW ratio is reportedly associated with blood cell components in patients with diabetes (5). This indicates that BIA may reflect blood components, although further validation is needed.
Indications for BIA have broadened, and it has been recognized as a reproducible measure for pediatrics (3,6). Children with nephrotic syndrome (NS) are no exception, with scattered reports of fluid assessment by BIA (7-11). However, these reports did not conduct longitudinal measurements. In addition, comparisons are set with healthy children or at arbitrary points in the acute phase. Therefore, it remains unclear what influenced the results and whether this technique is clinically applicable. To address this issue, longitudinal evaluation of the ECW/TBW ratio and factors, such as albumin, related to fluid composition is pertinent. Idiopathic NS (INS) involves the loss of podocytes or podocytopathy, resulting in massive proteinuria, including albumin (12). Albumin is the most abundant protein in extracellular fluid. It is vital for regulating fluid distribution in the human body and accounts for approximately 60% of plasma proteins (13). As albumin determines colloid osmotic pressure, hypoalbuminemia causes a decrease in effective circulating blood volume (14). Careful management is required because the progression of this condition may lead to acute kidney injury (AKI) or hypovolemic shock (15). Fluid management is a crucial therapeutic approach in edematous idiopathic INS; albumin administration reduces the risk of AKI by maintaining the circulating blood volume and enhances the effects of furosemide (16). While knowledge of the S-Alb level is essential in fluid management, frequent blood testing is burdensome for children. Although fluid management is based primarily on a subjective clinical assessment, clinicians need noninvasive bedside tools that objectively evaluate fluid status (9). Thus, the ability to quantify S-Alb levels rapidly and noninvasively may contribute to improved fluid management in children with INS. Herein, we present a longitudinal study of a child with edematous INS. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-211/rc).
Case presentation
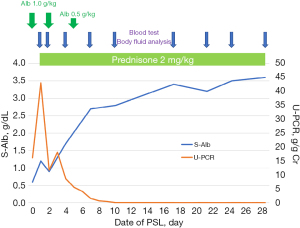
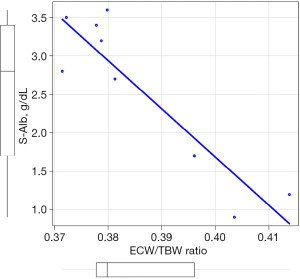
The patient was a 6-year-old boy with edema that persisted for several days. At the time of presentation, his height was 122.7 cm [+0.9 standard deviation (SD)], and his weight was 25.8 kg (+0.84 SD); an increase of 3 kg from before INS. His S-Alb level was 0.6 g/dL, and his urine protein/creatinine (Cr) ratio (U-PCR) was 16.14 g/g Cr. No pleural fluid, pancytopenia, low serum levels of complements, or abnormal antibodies were noted. The patient was diagnosed with INS and was started on prednisolone (PSL) 60 mg/m2. Proteinuria resolved on day 7 and remained in remission. Fluid management was required during treatment, and gastrointestinal symptoms, ascites, and edema were prominent; albumin was supplemented three times. Figure 1 shows the proteinuria outcomes, S-Alb levels, and treatment details. Following treatment, ascites and gastrointestinal symptoms were no longer observed on day 4 and day 6, respectively. Fluid volume measurements were performed nine times using an InBody S10 multi-frequency body water analyzer (InBody Japan Inc., Tokyo, Japan). Each measurement, which took approximately 10 minutes, was performed in a supine position within 2 hours following the morning blood test and more than 12 hours after the last albumin administration by a registered dietitian. The factors and time during measurement were consistent during the evaluation period. S-Alb levels were measured intermittently throughout the study. The correlation between the ECW/TBW ratio and S-Alb levels was determined using Spearman’s rank correlation coefficient, which showed a significantly strong negative correlation (r=−0.72, P<0.04) (Figure 2). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In this study, wherein fluid measurements were taken over time in a child with INS, the ECW/TBW ratio significantly correlated with the S-Alb level. The ECW/TBW ratio increased as fluid levels increased due to edema. Notably, the possibility of noninvasively ascertaining S-Alb levels, an important factor in INS management, has been demonstrated in this study.
Using a consistent measurement protocol allows for the accurate monitoring of fluctuations and can contribute to correctly adjusting the patient’s weight (3). This study was able to monitor S-Alb levels and conduct BIA over time by synchronizing each other. Knowledge of S-Alb levels may be useful in understanding nephrotic conditions and risk management. Hypoalbuminemia (<2.5 g/dL) is reportedly associated with a significantly lower glomerular filtration rate than non-hypoalbuminemia (≥2.5 g/dL) in minimal change NS (17). In pediatric INS, S-Alb levels were significantly lower in the AKI group than in the non-AKI group (1.68 vs. 2.06 g/dL) (18). In the non-remission period of pediatric NS, hypoalbuminemia (<3.5 g/dL) was associated with pulmonary embolism (19). Although these are retrospective studies, they suggest the possibility of appropriate intervention. The correlation between the ECW/TBW ratio and S-Alb levels observed in this study enable accurate fluid management in children with INS. Clinical evaluation alone cannot distinguish between ICW and ECW for determining hydration status in children (6). In contrast, bioimpedance spectroscopy can sensitively reflect ECW (20). Previous studies have shown that ECW was increased in INS with low S-Alb levels in the acute phase compared to those in remission or healthy children (7,8). Similarly, the ECW/TBW ratio was increased in INS at initial onset or relapse compared to those in remission or healthy children (9). As previous reports have only compared acute phase measurements at a single point during edema, it was necessary to clarify how the fluctuations from the acute phase would evolve; this was true in the present longitudinal study, with the ECW/TBW ratios negatively correlating with S-Alb levels. Moreover, previous studies involving patients with viral hepatitis have shown a significant negative correlation between S-Alb levels and the ECW/TBW ratio, justifying its potential for improving fluid management (21). In INS, children with decreased intravascular blood volume or severe diuretic refractory edema benefit from albumin administration (22). Albumin administration can be effective in inducing diuresis and reducing edema. However, the use of albumin and furosemide remains controversial (16,23), meaning administration should only be allowed in certain situations. Therefore, careful judgment is required in albumin administration. Although the determining factor for albumin administration is the condition of the affected child, such as edema, ascites, and gastrointestinal symptoms, management may be further improved by considering the S-Alb level. The prediction equation for the ECW/TBW ratio (= 0.3835 + 0,00118 × age) was derived from healthy volunteers, and the confidence interval was reportedly ±5% (24). At the time of this report, the standard value of the ECV/TBW ratio is 0.39058; this is consistent with the relationship between hypoalbuminemia (<2.5 g/dL) and the ECW/TBW ratio measured in the present case. This indirectly supports the ability of the ECW/TBW ratio to ascertain S-Alb levels, and it is possible that the ECW/TBW ratio could also be used to ascertain S-Alb levels noninvasively. This simple bedside measurement method is expected to reduce the burden on the patient.
The present study reports the continuous evaluation of a patient for approximately 1 month after the initial onset. Its main limitation is that only one patient was evaluated. In addition, BIA depends on a variety of factors, such as age, mineral content, and nutritional status (25). Hence, it is not possible to compare the measurements taken in this study with others, but it can be inferred that the variations observed in the same patient have a certain degree of reliability. In addition, brain natriuretic peptide levels and inferior vena cava diameter, which are indicators of fluid assessment, have not been measured synchronously with BIA to minimize the invasiveness of the measurement. These parameters and the robustness of this technique need to be verified with more cases in the future.
Conclusions
We proposed using InBody for fluid measurements to determine S-Alb levels from the ECW/TBW ratio as an adjunct to INS therapy. Although further validation is warranted, this simple and minimally invasive test may decrease the burden on children suffering from INS.
Acknowledgments
The authors thank the patient of the study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-211/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-211/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-211/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr 2004;23:1226-43. [Crossref] [PubMed]
- Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 2006;27:921-33. [Crossref] [PubMed]
- Dasgupta I, Keane D, Lindley E, et al. Validating the use of bioimpedance spectroscopy for assessment of fluid status in children. Pediatr Nephrol 2018;33:1601-7. [Crossref] [PubMed]
- Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys 2008;30:1257-69. [Crossref] [PubMed]
- Hori T, Nakamura S, Yamagami H, et al. Phase angle and extracellular water-to-total body water ratio estimated by bioelectrical impedance analysis are associated with levels of hemoglobin and hematocrit in patients with diabetes. Heliyon 2023;9:e14724. [Crossref] [PubMed]
- Frey SM, Vogt B, Simonetti GD, et al. Differential assessment of fluid compartments by bioimpedance in pediatric patients with kidney diseases. Pediatr Nephrol 2021;36:1843-50. [Crossref] [PubMed]
- Brantlov S, Jødal L, Andersen RF, et al. An evaluation of phase angle, bioelectrical impedance vector analysis and impedance ratio for the assessment of disease status in children with nephrotic syndrome. BMC Nephrol 2019;20:331. [Crossref] [PubMed]
- Drozdz D, Sancewicz-Pach K, Wierzchowska-Słowiaczek E. Value of bioelectrical impedance analysis in the assessment of body water in children with nephrotic syndrome: initial results. Pol Merkur Lekarski 2000;8:224-5. [PubMed]
- Nalcacioglu H, Ozkaya O, Baysal K, et al. The role of bioelectrical impedance analysis, NT-ProBNP and inferior vena cava sonography in the assessment of body fluid volume in children with nephrotic syndrome. Nefrologia (Engl Ed) 2018;38:48-56. [Crossref] [PubMed]
- Brantlov S, Jødal L, Frydensbjerg Andersen R, et al. Bioimpedance Resistance Indices and Cell Membrane Capacitance Used to Assess Disease Status and Cell Membrane Integrity in Children with Nephrotic Syndrome. ScientificWorldJournal 2019;2019:4274856. [Crossref] [PubMed]
- Özdemir K, Mir MS, Dinçel N, et al. Bioimpedance for assessing volume status in children with nephrotic syndrome. Turk J Med Sci 2015;45:339-44. [Crossref] [PubMed]
- Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet 2018;392:61-74. [Crossref] [PubMed]
- KEKWICK RA. MARTIN N. Plasma protein fractions. Proc R Soc Med 1948;41:217-24. [Crossref] [PubMed]
- Perico N, Remuzzi G. Edema of the nephrotic syndrome: the role of the atrial peptide system. Am J Kidney Dis 1993;22:355-66. [Crossref] [PubMed]
- Yang EM, Yoo KH, Ahn YH, et al. Lower albumin level and longer disease duration are risk factors of acute kidney injury in hospitalized children with nephrotic syndrome. Pediatr Nephrol 2021;36:701-9. [Crossref] [PubMed]
- Kallash M, Mahan JD. Mechanisms and management of edema in pediatric nephrotic syndrome. Pediatr Nephrol 2021;36:1719-30. [Crossref] [PubMed]
- Löwenborg EK, Berg UB. Influence of serum albumin on renal function in nephrotic syndrome. Pediatr Nephrol 1999;13:19-25. [Crossref] [PubMed]
- Sharma M, Mahanta A, Barman AK, et al. Acute kidney injury in children with nephrotic syndrome: a single-center study. Clin Kidney J 2018;11:655-8. [Crossref] [PubMed]
- Hoseiny Nejad N, Sharif AS, Otukesh H, et al. Determination of the value of albumin, anti-thrombin III, fibrinogen and D-dimer factors in the diagnosis of asymptomatic pulmonary embolism in patients with nephrotic syndrome. Pediatr Nephrol 2021;36:1803-8. [Crossref] [PubMed]
- Khin EE, Elmaghrabi AY, Alvarado LA, et al. Fluid balance assessment in pediatric hemodialysis patients by using whole-body bioimpedance spectroscopy (WB-BIS). Pediatr Nephrol 2022;37:2449-56. [Crossref] [PubMed]
- Nishikawa H, Yoh K, Enomoto H, et al. Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis. Nutrients 2018;10:1072. [Crossref] [PubMed]
- Meena J, Bagga A. Current Perspectives in Management of Edema in Nephrotic Syndrome. Indian J Pediatr 2020;87:633-40. [Crossref] [PubMed]
- Gipson DS, Massengill SF, Yao L, et al. Management of childhood onset nephrotic syndrome. Pediatrics 2009;124:747-57. [Crossref] [PubMed]
- Lopot F, Nejedlý B, Novotná H, et al. Age-related extracellular to total body water volume ratio (Ecv/TBW)--can it be used for "dry weight" determination in dialysis patients? Application of multifrequency bioimpedance measurement. Int J Artif Organs 2002;25:762-9. [Crossref] [PubMed]
- Chumlea WC, Schubert CM, Sun SS, et al. A review of body water status and the effects of age and body fatness in children and adults. J Nutr Health Aging 2007;11:111-8. [PubMed]
Cite this article as: Nishino T, Takahashi K, Ochiai C, Tomori S, Ono S, Mimaki M. Association between serum albumin and body water using a bioelectrical impedance analyzer: a case report of longitudinal variation in a child with initial idiopathic nephrotic syndrome. AME Case Rep 2024;8:62.


