
Metaplastic thymoma in the middle mediastinum: a rare case report and surgical treatment analysis of a 32-year-old female patient
Highlight box
Key findings
• Our findings highlight a rare case of metaplastic thymoma (MT) in a young female, unusual due to its middle mediastinal location rather than the common anterior positioning.
What is known and what is new?
• Currently, thymomas are understood as tumors originating from the epithelial cells of the thymus, typically presenting in the anterior mediastinum. They are classified based on their histological appearance, ranging from well-differentiated to more aggressive forms. MT, a rare subtype, is distinguished by its unique histopathological features, including a mix of epithelioid and spindle cells. It tends to have an indolent course, with surgical resection often being curative.
• Typically, thymomas occur in older patients and predominantly in the anterior mediastinum. This case’s novelty lies in the patient’s young age, the tumor’s unusual middle mediastinal location, and the pathology of a rare MT.
• While the YAP1::MAML2 gene fusion is not a novel discovery in itself, its identification in this atypical context underscores the genetic diversity of thymomas and provides additional validation of its role.
What is the implication, and what should change now?
• This case highlights the need for heightened awareness of MT in atypical presentations and supports the inclusion of genetic testing for YAP1/MAML2 fusion in the diagnostic process, potentially influencing treatment strategies for rare thymomas.
Introduction
Metaplastic thymoma (MT), an exceedingly rare thymic neoplasm, is infrequently encountered in clinical practice, resulting in limited comprehensive understanding (1-6). This entity often presents diagnostic and therapeutic conundrums, attributed to its non-specific clinical and radiological manifestations.
MT often presents with non-specific clinical and radiological features. The majority of patients are initially identified via imaging studies, yet definitive diagnosis invariably requires comprehensive histopathological evaluation. Given its predominantly indolent course, it is imperative for clinicians to be acquainted with the nuances of this rare pathology to ensure prompt, accurate diagnosis and surgical intervention. Although surgical resection is typically the preferred modality for treating MT, patient receptivity may be limited. This hesitancy is often attributed to the tumor’s slow growth and smooth margins as evidenced on imaging.
MT is an uncommon biphasic tumor, distinguished by its unique pathological morphology. The tumor is composed of benign spindle cells intermixed with solid epithelial cells. These two components can display either an abrupt transition or a gradual change. Grossly, the tumor is well-demarcated or appears encapsulated, but some areas may exhibit invasive budding. The tumor cells exhibit bidirectional differentiation, with the epithelial-like cells and spindle cells either sharply demarcated or gradually transitioning. The proportion of these components varies (7). Vivero et al. have elucidated the presence of the YAP1::MAML2 fusion gene within MT, setting it apart from the prevalent GTF2I gene mutations observed in type A or AB thymomas (8).
This report presents a rare case of MT in a 32-year-old female patient, highlighting the diagnostic challenges and the critical need for early and accurate diagnosis. We comprehensively detail the patient’s clinical presentation, diagnostic journey, and surgical treatment, discussing the implications of these findings for future clinical practice. This case aims to deepen the understanding of MT, particularly its manifestation in young females and within middle mediastinal tumors, thereby fostering further research and discourse on this rare condition. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-213/rc).
Case presentation
During a routine lung cancer screening at a health examination center, a chest computed tomography (CT) scan revealed a mass in the middle compartment of the left mediastinum in a 32-year-old woman. The patient was asymptomatic preoperatively, exhibiting no cough, expectoration, dyspnea, chest discomfort, or chest pain. There were no signs of myasthenia gravis (MG) or other related symptoms. Preoperative testing for anti-acetylcholine receptor antibodies was conducted, with results returning negative. The patient’s medical history, medication history, psychiatric history, family history, and social and occupational background were thoroughly reviewed. No abnormalities were identified upon examination. A follow-up chest CT indicated a well-defined, hyperdense nodule in the left middle mediastinum, with a CT value of approximately 32 Hounsfield units (HU). The demarcation from adjacent pericardium was indistinct, prompting recommendation for a contrast-enhanced CT. The enhanced chest CT revealed significant heterogeneous enhancement of the nodule, suggesting a high likelihood of a benign lesion (Figure 1). The radiological features of this mediastinal mass, including its morphology and density, exhibit atypical characteristics when compared to those commonly observed in mediastinal entities, including bronchogenic cysts, pericardial cysts, and neoplasms such as lymphomas. These unique imaging findings introduce complexities in the preoperative diagnostic process. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
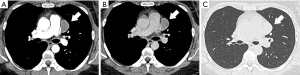
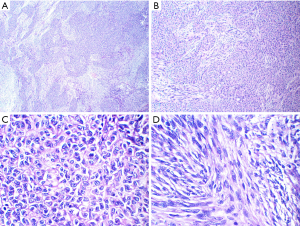
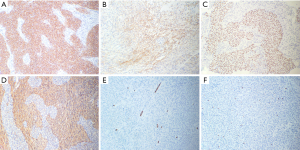
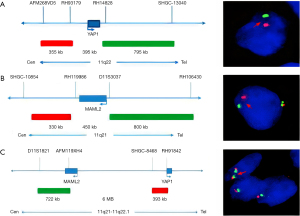
Given the tumor’s well-defined margins and absence of invasion into adjacent critical structures, we selected a minimally invasive single-port video-assisted thoracoscopic surgery (VATS) technique. Anesthesia was achieved through double-lumen intubation, facilitating intraoperative single-lung ventilation, and CO2 insufflation was not utilized. The surgical procedure was completed in 35 minutes. On exploration, the tumor exhibited clear demarcation and favorable mobility, prompting its resection alongside adjacent adipose tissue without the need for an extended thymectomy. Notably, the procedure carefully addressed the tumor’s proximity to the phrenic nerve, which was meticulously preserved without any compromise. Macroscopically, the tumor was nearly oval, measuring 3 cm × 2.5 cm × 2 cm, with a capsule-like surface, solid cut surface, grey-white in color, and firm in texture. Microscopic examination revealed a tumor composed of epithelioid and spindle cells. The epithelioid cells were arranged in islands and sometimes in trabecular patterns. They were oval or polygonal with eosinophilic cytoplasm. Spindle cells, resembling fibroblasts and characterized as bland, non-atypical, and benign-looking, were interspersed between these islands. No mitotic figures were observed (Figure 2). Immunohistochemistry demonstrated strong positivity for CK-pan and P40 in epithelial cells, with spindle cells showing weak and focal positivity for epithelial membrane antigen (EMA) and strong positivity for vimentin. Neither cell type expressed CD34, and the Ki-67 index was less than 5% (Figure 3). The subject underwent fluorescence in situ hybridization (FISH) analysis to identify the YAP1::MAML2 gene fusion, utilizing a bi-color FastProbe specifically tailored for this detection (Kanglu Medical Laboratory, Wuhan, China). The assay utilized an orange-red (R) signal for the YAP1 gene’s 5' terminus and a green (G) signal for the 3' end of the MAML2 gene. Normal cellular fluorescence is characterized by a 2R2G signal configuration, while the presence of a fusion gene is typically indicated by a 1F1R1G pattern. Analysis of a cohort of 100 cells revealed the following: 26% exhibited 1F signals, indicative of fusion; 6% presented with 2F signals; another 6% displayed 1R1G signals; a significant 58% showed the 1F1R1G pattern, strongly suggesting the presence of the fusion gene; and a minority of 4% maintained a normal 2R2G signal pattern. These findings conclusively indicate a positive YAP1::MAML2 fusion gene FISH status in the sample (Figure 4). These findings were consistent with a diagnosis of MT.
The patient demonstrated a favorable postoperative recovery trajectory, facilitating discharge from the hospital on the fifth day following the surgical intervention. Subsequent outpatient follow-up, including a chest direct radiography (DR) conducted two weeks post-surgery, revealed no significant accumulation of pleural fluid, indicating a satisfactory respiratory status. Additionally, the surgical site exhibited optimal healing post-suture removal. The postoperative pathology report confirmed that the resection margins were negative, and classified the tumor as Masaoka stage I. Considering the early stage of the disease and the successful completion of an R0 resection, further adjuvant radiotherapy was deemed unnecessary. The patient is currently engaged in a regular follow-up regimen in the outpatient setting, 6 months post-tumor excision, to monitor ongoing recovery and detect any potential complications at an early stage.
Discussion
MT is a rare biphasic tumor characterized by distinctive pathological morphology. It comprises mild spindle cells and interspersed solid epithelial cells, exhibiting transitions ranging from abrupt to gradual. Accounting for less than 1% of all thymoma types, MT has been primarily reported in limited case studies or small series, with patient ages ranging from 28 to 71 years and no significant gender disparity in incidence. Initially described in 1997 by Suster et al. as “thymoma with pseudosarcomatous stroma”, they posited that only epithelioid cells were neoplastic, while spindle cells were reactive (9). In 1999, Yoneda et al. analyzed five cases and observed transitions between these two cell types. Both components expressed epithelial markers, suggesting a neoplastic origin from pluripotent stem cells (10). The World Health Organization (WHO) officially named it MT in 2004, categorizing it as a tumor with malignant potential or of borderline malignancy, coded as 8580/1 in the ICD-O. The WHO’s 2015 update on thymic tumors reflected a deeper understanding of thymoma’s biological behavior, reclassifying MT along with conventional thymomas (type A, AB, and B) as per the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), assigning them a behavior code of 3 to indicate malignancy. MT has been assigned the code XH3DX0 in the International Classification of Diseases 11th Revision (ICD-11) (11). This classification underscores the recognition and distinct categorization of MT within the framework of global health diagnostics. This case report describes a rare instance of a 32-year-old female patient with MT, an uncommon thymic tumor, uniquely located not in the usual anterior mediastinum but in the middle mediastinum adjacent to the phrenic nerve.
In our manuscript, we present a novel case of MT uniquely situated in the middle mediastinum, diverging from the common anterior mediastinal presentation of such tumors. This report appears to be the inaugural documentation of MT in this atypical locale, according to our comprehensive review. Despite the tumor’s uncommon positioning, the preoperative assessments, surgical exploration, and postoperative recovery unfolded in a standard manner, indicating that the unusual location did not necessitate deviation from established clinical protocols. On CT, MT typically manifests as a well-demarcated, homogeneously or slightly heterogeneously dense mass within the anterior mediastinum. It may exhibit mild to moderate enhancement following intravenous contrast, with potential internal calcifications, cystic transformations, or necrotic zones due to the tumor’s mixed cellular composition. However, these imaging characteristics are not unique to MT, representing a spectrum of radiological features associated with the disease
MT is named for its cellular differentiation diversity and often involves complex diagnostic processes. The diagnosis of MT relies on the combination of histological and immunohistochemical characteristics. In this case, the cellular diversity observed under the microscope and the specific expression pattern of immunohistochemical markers align with the characteristics of MT. In our immunohistochemical analysis, distinct patterns of marker protein expression delineate the cellular composition of the tumor, thereby reinforcing the diagnostic framework. Specifically, epithelial cell compartments are unequivocally marked by the presence of CK (pan) and P40, affirming their epithelial lineage. Conversely, a proliferation index, as denoted by Ki-67, remains subdued below the 10% threshold across the tumor landscape, indicating a relatively indolent proliferative demeanor. Furthermore, the expression of Vimentin within the stromal cells underscores their mesenchymal genesis, completing a comprehensive profile of tumor histopathology. Despite the critical role of histological features and immunohistochemistry in diagnosing MT, its clinical and radiological presentations are not specific, often leading to diagnostic delays. In this case, the patient was asymptomatic, and the tumor was incidentally discovered during routine examination, highlighting the importance of early detection and diagnosis.
The primary treatment for MT was surgical resection, executed successfully through VATS without adjuvant therapy. Such an approach is prevalent in clinical practice, yet, due to the rarity of MT, definitive treatment protocols remain unestablished. The indeterminate recurrence rate and metastatic potential of this neoplasm underscore the importance of regular follow-up and radiological monitoring for early detection of recurrence. Given the short postoperative period in this case, surgical cure cannot be conclusively assured. Considering the potential invasiveness of the tumor, prolonged clinical and radiological surveillance is recommended.
Conclusions
This case emphasizes the diagnostic complexity and rarity of MT, particularly within the middle mediastinum, a novel presentation for such tumors. Our comprehensive approach, combining histopathological examination, immunohistochemical analysis, and FISH for YAP1::MAML2 gene fusion, was pivotal in achieving a definitive diagnosis. Surgical resection remained the cornerstone of treatment, underscoring the importance of early detection and precise surgical intervention. This report contributes significantly to the limited literature on MT, highlighting the need for awareness and further research into this unique thymic epithelial tumor.
Acknowledgments
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-213/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-213/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-213/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han L, Gao B, Wang EH, et al. Giant Multilocular-Cystic Metaplastic Thymoma: A Case Report. Pharmgenomics Pers Med 2023;16:463-6. [Crossref] [PubMed]
- Fu R, Zhang YT, Li XH. A rare case of thymic epithelial tumor: Metaplastic thymoma. Asian J Surg 2022;45:1044-5. [Crossref] [PubMed]
- Komori K, Tsubochi H, Ohno K, et al. Metaplastic Thymoma Underwent Surgery:Report of a Case. Kyobu Geka 2021;74:241-3. [PubMed]
- Tajima S, Yanagiya M, Sato M, et al. Metaplastic thymoma with myasthenia gravis presumably caused by an accumulation of intratumoral immature T cells: a case report. Int J Clin Exp Pathol 2015;8:15375-80. [PubMed]
- Lu HS, Gan MF, Zhou T, et al. Sarcomatoid thymic carcinoma arising in metaplastic thymoma: a case report. Int J Surg Pathol 2011;19:677-80. [Crossref] [PubMed]
- Poorabdollah M, Mehdizadeh E, Mohammadi F, et al. Metaplastic thymoma: report of an unusual thymic epithelial neoplasm arising in the wall of a thymic cyst. Int J Surg Pathol 2009;17:51-4. [Crossref] [PubMed]
- Jin M, Liu B, Wang L, et al. Clinicopathologic study of metaplastic thymoma. Zhonghua Bing Li Xue Za Zhi 2006;35:285-8. [PubMed]
- Vivero M, Davineni P, Nardi V, et al. Metaplastic thymoma: a distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions. Mod Pathol 2020;33:560-5. [Crossref] [PubMed]
- Suster S, Moran CA, Chan JK. Thymoma with pseudosarcomatous stroma: report of an unusual histologic variant of thymic epithelial neoplasm that may simulate carcinosarcoma. Am J Surg Pathol 1997;21:1316-23. [Crossref] [PubMed]
- Yoneda S, Marx A, Müller-Hermelink HK. Low-grade metaplastic carcinomas of the thymus: biphasic thymic epithelial tumors with mesenchymal metaplasia--an update. Pathol Res Pract 1999;195:555-63. [Crossref] [PubMed]
- Marx A, Chan JKC, Chalabreysse L, et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J Thorac Oncol 2022;17:200-13. [Crossref] [PubMed]
Cite this article as: Wang Z, Zong W, Liang S, Sun D. Metaplastic thymoma in the middle mediastinum: a rare case report and surgical treatment analysis of a 32-year-old female patient. AME Case Rep 2024;8:64.


