
Epithelioid hemangioendothelioma: a case report
Highlight box
Key findings
• Epithelioid hemangioendothelioma (EHE) is a rare vascular tumor with limited clinical data to guide treatment selection. In the present case, surveillance was conducted and the patient’s clinical course was better than expected.
What is known and what is new?
• The prognosis of EHE is variable.
• This case has been demonstrated an indolent clinical course.
What is the implication, and what should change now?
• The treatment for EHE should be selected according to the patient’s condition.
Introduction
The term “epithelioid hemangioendothelioma” (EHE) was first described as a distinct entity by Weiss and Enzinger in 1982 (1). They presented a study of 41 soft tissue vascular tumors with unpredictable courses. EHE is a rare neoplasm of vascular origin that may develop at different sites, such as the soft tissue, lungs, or liver. It usually affects adult women, and its malignant potential ranges from benign to hemangioendotheliosarcoma. Primary liver involvement was first reported in 1984 by Ishak et al. (2) among 32 primary hepatic hemangioendotheliomas. EHE is characterized by epithelioid or histiocytoid morphology and a growth pattern with evidence of endothelial histogenesis (3).
In general, the neoplasm has a protracted, relatively benign clinical course, intermediate between benign hemangioma and malignant hemangioendotheliosarcoma. The patients reported nonspecific symptoms such as right upper quadrant or epigastric discomfort or pain (52.8%), weight loss (24.4%), and weakness (11.8%). Less common symptoms at initial presentation were jaundice (8.7%), fever (7.9%), and fatigability (5.5%). The most frequent signs observed on ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI) were hepatomegaly (45.7%) and splenomegaly (17.3%). Ascites (12.6%) and portal hypertension (4.7%) were rarely observed, whereas increased serum alkaline phosphatase levels were observed in 70% of the patients. Other liver enzymes could not be assessed because the data were omitted by various authors. Serum α-fetoprotein (AFP) levels were elevated in only 2.7% of patients, and no elevated levels of the tumor markers carcinoembryonic antigen (CEA) and cancer antigen (CA) 19-9 were found (3). Ishak et al. (2) described metastatic disease in approximately 28% of patients with EHE of the liver, with preferential involvement of the regional lymph nodes, liver, lungs, peritoneum, and retroperitoneum. The overall rate of metastasis was 45.1%, of which 19.7% was in the lungs, 9% in the bones, 4.9% in the spleen, and 21.3% in other organs such as the heart, retroperitoneum, or brain. Regional and extrahepatic lymph nodes were involved in 15.8% and 8.2% of the patients, respectively. The age of the patients at presentation ranged from 12 to 86 years (mean, 39.8 years) (4). We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-51/rc).
Case presentation
A 62-year-old woman with weight loss and general weakness was admitted to the Ajou University Hospital. The patient was well until three months before admission, when malaise, fatigue, and weight loss (loss of 10 kg over one year) developed, accompanied by sleep disturbance and stress. The patient had a family history of myocardial infarction. Additionally, she had sustained a lumbar fracture after falling out of bed 7 months prior and had been admitted to the orthopedic clinic for 2 weeks. She had no history of any other medical condition and did not smoke or use illicit drugs. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
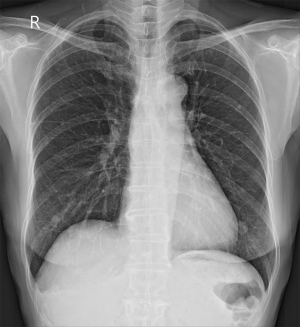
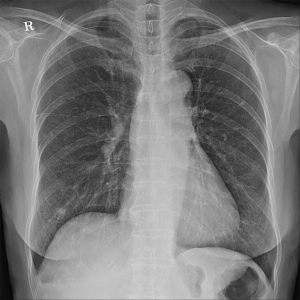
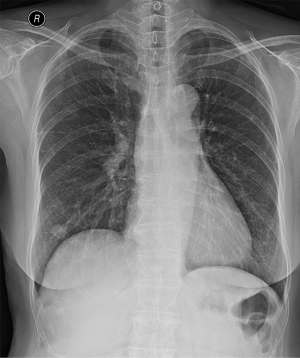
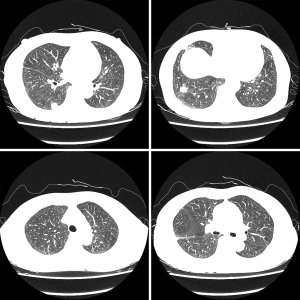
Upon examination, her blood pressure was 108/60 mmHg, her pulse rate was 92 beats/min, her height was 169 cm, and her weight was 55.8 kg. She appeared slightly ill and mildly distressed because of the weight loss. Her blood glucose, electrolyte, and creatine kinase levels were normal. The complete blood count, differential count, and renal and liver function test results were within the normal ranges. Urinalysis revealed clear yellow urine with a specific gravity of 1.024 and a pH of 6.0, with no glucose, ketones, bilirubin, protein, blood, or nitrates. Sediment examination revealed no red or white cells, bacteria, or casts. The C-reactive protein level was <0.03 mg/dL (reference range, 0–0.5 mg/dL); erythrocyte sedimentation rate, 2 mm/h (reference range, <25 mm/h); serum cortisol level, 7.3 µg/dL; and serum dehydroepiandrosterone sulfate (DHEA-S), 47 µg/dL. All tumor markers, including AFP, CEA, and Ca-125 were within normal limits. No positive markers of hepatitis A or B were detected. Chest radiography revealed multiple variably sized nodules in both lungs (Figure 1).
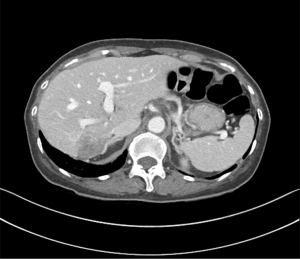
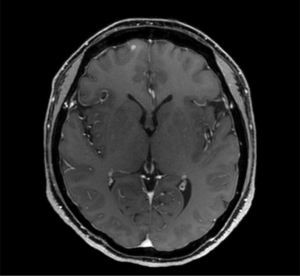
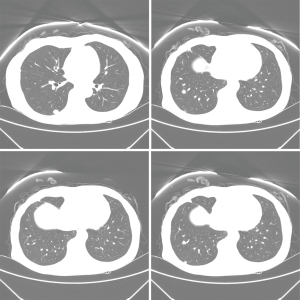
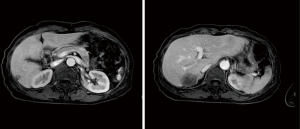
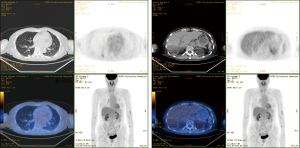
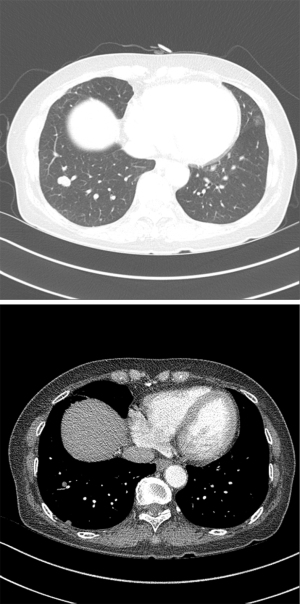
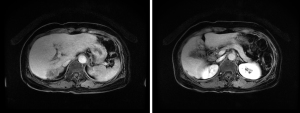
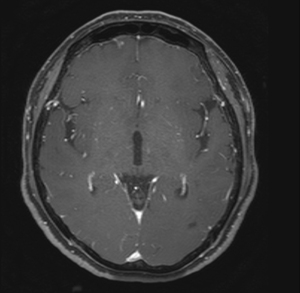
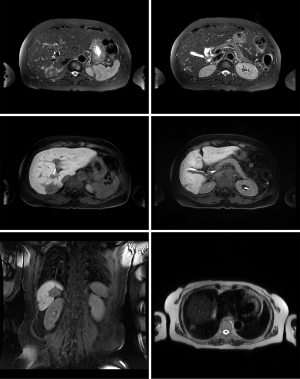
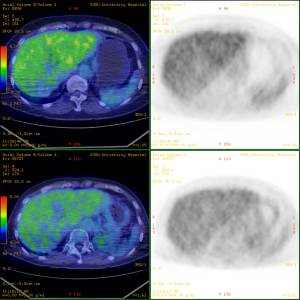
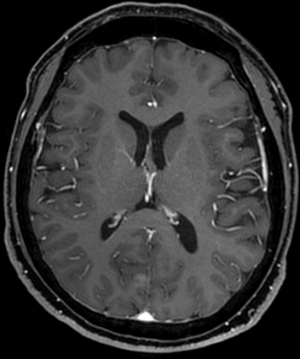
Abdominal CT revealed approximately 1.3- and 3.4-cm sized progressively enhanced masses in size (Figure 2). The brain MRI and chest CT findings are shown in Figure 3 and Figure 4, respectively. Ten days later, she was readmitted for liver MRI and positron emission tomography (PET) CT (Figures 5,6).
Differential diagnosis
Liver MRI revealed a 4.4 cm peripherally enhanced mass and 1.1 cm nodular lesion in the right posterior segment of the liver with similar characteristics, along with subtle T2 high signal intensity and diffusion restriction with mild capsular retraction. The differential diagnoses included cholangiocarcinoma, metastasis, and EHE.
Cholangiocarcinoma
Macroscopically, three patterns of cholangiocarcinoma growth have been described (5,6): (I) mass-forming (exophytic), which results in a definite mass in the liver parenchyma; (II) infiltrating (periductal), which extends longitudinally along the bile duct, often resulting in dilatation of the peripheral ducts, and is either nodular or diffusely infiltrating (7); and (III) polypoidal (intraductal) growth pattern, which proliferates towards the bile duct lumen in the form of papillae or tumor thrombus. Intrahepatic cholangiocarcinomas are typically mass-forming and perihilar, whereas extrahepatic cholangiocarcinomas are mostly infiltrating; however, these rarely present with a polypoidal growth pattern. Combined cholangiocarcinomas encompassing more than one growth type are commonly observed in intrahepatic tumors.
Liver metastasis
Liver metastases may be hypovascular or hypervascular. Hypovascular liver metastases most commonly originate from colon, lung, breast, and gastric carcinomas which typically exhibit perilesional enhancement. Neuroendocrine tumors, including carcinoid and islet cell tumors, renal cell carcinoma, breast cancer, melanoma, and thyroid carcinoma, most commonly cause hypervascular liver metastases, which may develop early enhancement with variable degrees of washout and peripheral rim enhancement (8).
EHE
EHE lesions are of three types, predominantly located in submarginal areas. On contrast-enhanced MRI, the findings for the first two types include a peripheral ring-like enhancement with a central low signal intensity (“black target-like” sign) and a central enhanced core surrounded by a low signal intensity halo (“white target-like’’ sign). CT and MRI findings for the third hepatic EHE type (diffuse type) include low density or heterogeneous signal intensity lesions involving specific regions or the whole liver, with coalescent lesions (“strip-like” sign) and gradual enhancement along central vessels (“lollipop” sign) (9).
Pathological diagnosis
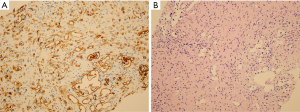
Fine-needle aspiration biopsy confirmed the diagnosis of EHE (December 21, 2016) (Figure 7). The specimen consisted of two fragments of elongated yellow-brown needle-biopsied liver tissue measuring up to 1.2 cm. Immunostaining of the tumor was positive for CD34 and negative for cytokeratin 7. Histological findings showed an increased vascular structure and infiltration of single or small clusters of atypical epithelioid endothelial cells in a fibromyxoid background. These tumor cells showed intravascular polypoid growth and were positive for CD34 expression (Figure 7A,7B).
After considering chemotherapy and surveillance, the patient opted for surveillance because surgery was not recommended at that time. She consumed a healthy vegetable-rich diet, exercised regularly, and attempted to remain mentally calm.
EHE is an uncommon vascular tumor with intermediate malignant potential (10). Hepatic EHE, a rare sarcoma of the liver, usually appears as multiple nodules involving both hepatic lobes, and can be misdiagnosed as a metastatic carcinoma based on its radiologic manifestations. Hepatic EHEs are composed of neoplastic endothelial cells that closely resemble epithelial cells and exhibit a characteristic zoning. At the periphery, tumor cells infiltrate the preexisting sinusoids and terminal hepatic venules. The center reveals a marked desmoplastic stromal reaction with dense sclerosis; these findings mimic those of cholangiocarcinoma. Therefore, confirming that the tumor is of vascular origin by immunohistochemical staining for endothelial cell markers is critical for the accurate diagnosis of hepatic EHE (11). Immunohistochemical reactivity to factor VIII-related antigens, cytokeratin, and CD 34 has been detected in endothelial cells (12). CD34, a hematopoietic progenitor antigen, is a sensitive marker of vascular neoplasms including EHE (13). The key to diagnosing EHE is the identification of cells containing factor VIII-related antigen, which confirms the endothelial origin of the tumor and is found in almost all patients (97.5%).
The patient underwent a follow-up examination 2.5 years later, wherein she was asymptomatic with a healthy general appearance. Blood glucose, electrolyte, and creatine kinase levels were normal. The complete blood count, differential count, and renal and liver function test results were within the normal ranges. The urinalysis yielded normal findings. The C-reactive protein level was 0.04 mg/dL; erythrocyte sedimentation rate, 8 mm/h; serum cortisol level, 12.7 µg/dL; and DHEA-S level, 105 µg/dL. All tumor markers (AFP, CEA, and Ca-125) were within normal limits.
Chest radiography revealed no changes in the right lower lung zone (RLLZ) nodular opacity (Figure 8), whereas chest CT revealed a few metastatic nodules that had increased in size since December 13, 2016. No hilar or mediastinal lymph node enlargement was observed; however, a mass was still present in the right lobe of the liver (Figure 9). Liver MRI revealed no significant interval changes in the peripherally enhancing masses in the right posterior segment of the liver, causing mild capsular retraction. No pathological lymph nodes were observed in the abdomen (Figure 10). Brain MRI showed no evidence of brain metastasis. The suspicious T2 low-signal intensity change in both dentate nuclei was suggestive of iron deposition; hence, it was ruled out (Figure 11).
At the subsequent follow-up conducted after 2.5 years, the patient was asymptomatic and had a healthy general appearance. blood glucose, electrolyte, and creatine kinase levels were normal. The complete blood count, differential count, renal, and liver function test results were within normal ranges; however, the total protein level decreased to 6.5 g/dL (reference range, 6.6 to 8.7 g/dL). The urinalysis results were normal. Her C-reactive protein level was 0.04 mg/dL, her erythrocyte sedimentation rate was 4 mm/h, her serum cortisol level was 8.8 µg/dL, and her DHEA-S level was 84 µg/dL. Her insulin-like growth factor-1 level was 60 ng/mL (reference range, 94–269 ng/mL). All tumor markers (AFP, CEA, and CA-125) were within normal limits.
Chest radiography revealed a 1.5 cm lung mass in the RLLZ (Figure 12). Chest CT revealed that the metastatic nodules in the right lower lobe had increased in size (from 1.6 to 2.2 cm, and from 1.5 to 1.8 cm) from May 6, 2019 (Figure 13). Liver MRI revealed no significant interval change in the size of the two peripherally enhancing masses in the right posterior segment of the liver, causing mild capsular retraction. No pathological abdominal lymph nodes were observed (Figure 14). In addition, PET-CT showed no significant interval changes in the isometabolic mass of the right liver lobe. However, the metastatic nodules in the right lung intermittently increased in size, with mildly increased metabolic activity, suggesting progressive metabolic disease (Figure 15). The previously recorded small enhancing lesion in the right frontal lobe disappeared on brain MRI (Figure 16). No specific evidence of skeletal metastasis was observed on a bone scan (Figure 17).
Discussion
EHE is a rare vascular tumor for which limited clinical data are available to guide its treatment. Disease presentations can be diverse and may include bone pain, neurological symptoms, and swelling that reflect the disease site. Patients occasionally present with systemic manifestations such as weight loss and anemia (14,15). EHE commonly presents as liver disease alone or with lung disease (14-16). It can also affect numerous other primary sites, such as the subcutaneous fat, bone, retroperitoneum, lymph nodes, ovaries, prostate, eyelids, and pleura. However, the blood workup findings are often normal (14).
Hepatic EHE commonly has a multifocal nodular presentation, with CT demonstrating clear tumor margins, centripetal enhancement in the arterial phase, and homogeneous enhancement in the portal venous and delayed phases. Peripheral ring enhancement with low signal intensity (the black target-like sign) is a typical radiographic finding on MRI (9).
The diagnosis of EHE is based on its unique histological, immunohistochemical, and molecular characteristics. The differential diagnoses include broad and include autoimmune granulomatous diseases (sarcoidosis and polyangiitis with granulomatosis), infections, and vascular malignancies (epithelioid angiosarcoma, epithelioid tumors, malignant mesothelioma, and melanoma) (14). Its treatments include hepatic resection, liver transplantation, systemic/regional chemotherapy, and radiotherapy (10,17-19).
The prognosis of EHE is variable: some cases demonstrate an indolent clinical course, whereas others tend to metastasize. The risk factors for worse outcomes include constitutional symptoms, such as weight loss and anemia; pulmonary symptoms, such as hemoptysis and hemorrhagic pleural effusions (20,21); and increased mitotic activity and size (22). In the national primary hepatic vascular malignancies (PHVM) cohort, tumor biology in the form of angiosarcoma histology, tumor differentiation, and tumor size were strongly associated with worse postoperative survival (23).
Conclusions
In our case, hepatic EHE had metastasized to the lungs and brain. The patient complained of weight loss and general weakness. Surveillance was conducted, and the clinical course was better than expected, probably due to her relatively good general condition, lack of genetic factors associated with her familial medical history, and normal levels of tumor markers such as AFP and CEA. Histopathological examination of the liver tissue revealed an epithelial hemangioendothelioma. On CK7 staining, hepatocytes were clearly reactive and were arranged in plates (CK7: negative), with positive immunohistochemical staining for CD34 (CD34: positive) alone. The patient underwent a follow-up examination during which she was asymptomatic with a healthy general appearance.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-51/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-51/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-51/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982;50:970-81. [Crossref] [PubMed]
- Ishak KG, Sesterhenn IA, Goodman ZD, et al. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol 1984;15:839-52. [Crossref] [PubMed]
- Läuffer JM, Zimmermann A, Krähenbühl L, et al. Epithelioid hemangioendothelioma of the liver. A rare hepatic tumor. Cancer 1996;78:2318-27. [Crossref] [PubMed]
- Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer 1999;85:562-82. [Crossref] [PubMed]
- Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288-91. [Crossref] [PubMed]
- Lee WJ, Lim HK, Jang KM, et al. Radiologic spectrum of cholangiocarcinoma: emphasis on unusual manifestations and differential diagnoses. Radiographics 2001;21 Spec No:S97-S116. [Crossref] [PubMed]
- Ahrendt SA, Nakeeb A, Pitt HA. Cholangiocarcinoma. Clin Liver Dis 2001;5:191-218. [Crossref] [PubMed]
- Namasivayam S, Martin DR, Saini S. Imaging of liver metastases: MRI. Cancer Imaging 2007;7:2-9. [Crossref] [PubMed]
- Gan LU, Chang R, Jin H, et al. Typical CT and MRI signs of hepatic epithelioid hemangioendothelioma. Oncol Lett 2016;11:1699-706. [Crossref] [PubMed]
- Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 2006;107:2108-21. [Crossref] [PubMed]
- Choi KH, Moon WS. Epithelioid hemangioendothelioma of the liver. Clin Mol Hepatol 2013;19:315-9. [Crossref] [PubMed]
- Ramani P, Bradley NJ, Fletcher CD. QBEND/10, a new monoclonal antibody to endothelium: assessment of its diagnostic utility in paraffin sections. Histopathology 1990;17:237-42. [Crossref] [PubMed]
- Traweek ST, Kandalaft PL, Mehta P, et al. The human hematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol 1991;96:25-31. [Crossref] [PubMed]
- Sardaro A, Bardoscia L, Petruzzelli MF, et al. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014;8:259. [Crossref] [PubMed]
- Bagan P, Hassan M, Le Pimpec Barthes F, et al. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg 2006;82:2010-3. [Crossref] [PubMed]
- Larochelle O, Périgny M, Lagacé R, et al. Best cases from the AFIP: epithelioid hemangioendothelioma of bone. Radiographics 2006;26:265-70. [Crossref] [PubMed]
- Wang LR, Zhou JM, Zhao YM, et al. Clinical experience with primary hepatic epithelioid hemangioendothelioma: retrospective study of 33 patients. World J Surg 2012;36:2677-83. [Crossref] [PubMed]
- Lin J, Ji Y CT. Hepatobiliary Pancreat Dis Int 2010;9:154-8. [PubMed]
- Grotz TE, Nagorney D, Donohue J, et al. Hepatic epithelioid haemangioendothelioma: is transplantation the only treatment option? HPB (Oxford) 2010;12:546-53. [Crossref] [PubMed]
- Amin RM, Hiroshima K, Kokubo T, et al. Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and a case report. Respirology 2006;11:818-25. [Crossref] [PubMed]
- Rosengarten D, Kramer MR, Amir G, et al. Pulmonary epithelioid hemangioendothelioma. Isr Med Assoc J 2011;13:676-9. [PubMed]
- Deyrup AT, Tighiouart M, Montag AG, et al. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol 2008;32:924-7. [Crossref] [PubMed]
- Dogeas E, Mokdad AA, Bhattatiry M, et al. Tumor Biology Impacts Survival in Surgically Managed Primary Hepatic Vascular Malignancies. J Surg Res 2021;264:481-9. [Crossref] [PubMed]
Cite this article as: Park SB, Kim YB, You S. Epithelioid hemangioendothelioma: a case report. AME Case Rep 2024;8:65.


