
Veno-venous or veno-arterial extracorporeal membrane oxygenation support for massive pulmonary embolism: a case report
Highlight box
Key findings
• Veno-venous extracorporeal membrane oxygenation (V-V ECMO) could be effective for some patients with massive pulmonary embolism (MPE) who suffer from successful cardiopulmonary resuscitation after cardiac arrest while still combined with severe hypotension and refractory hypoxemia.
What is known and what is new?
• Veno-arterial extracorporeal membrane oxygenation (V-A ECMO) is usually used as a therapy for severe shock and V-V ECMO is typically used in patients with severe refractory hypoxemia.
• We found that V-V ECMO could be effective for some patients with MPE combined with severe hypotension and hypoxemia through relieving abnormal vasoconstriction.
What is the implication, and what should change now?
• For some MPE patients with refractory respiratory failure and hypotension, V-V ECMO could be effective, so that V-A ECMO could be avoided, which could decrease the risk of the lower limb ischemia.
Introduction
The acute pulmonary embolism (APE), along with sustained hypotension, precisely, the systolic blood pressure (BP) was under 90 mmHg or lost more than 40 mmHg compared to baseline, lasting for more than 15 minutes without additional causes, could be defined as a massive pulmonary embolism (MPE) (1,2). Mostly, for patients with contraindications to reperfusion therapy and complicated with hemodynamic compromise for cardiogenic shock or cardiac arrest, veno-arterial extracorporeal membrane oxygenation (V-A ECMO), can bridge to specific embolectomy and thrombolysis (3-5). Veno-venous (V-V) ECMO is typically used in patients with severe hypoxia or hypercapnia (4). However, there are several reports about successful treatment in pulmonary embolism with V-V ECMO (6,7). This case reports an effective life-saving with V-V ECMO in MPE. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-128/rc).
Case presentation
A 35-year-old pregnant woman suddenly presented with complaints of nausea, vomiting and dyspnea after going to the toilet. Before that, she had been diagnosed with gestational diabetes mellitus for unknown time, and was hospitalized because of the threatening labor. She was given intensive insulin therapy, dexamethasone to promote fetal lung maturity and tocolysis to suppress uterine contraction for five days.
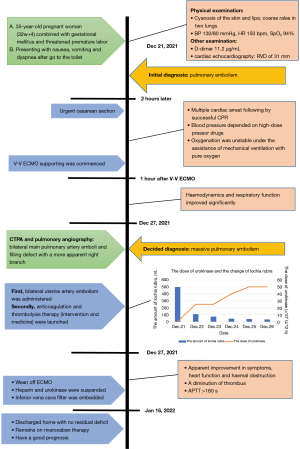
Enroute, on physical examination, the patient became cyanotic and coarse rales could be heard in two lungs. Her BP was 130/90 mmHg with an accelerated heart rate (HR) of 150 bpm, pulse oxygen saturation (SpO2) was 94% under treatment with oxygen mask, and D-dimer increased to 11.2 µg/mL. On point-of-care cardiac echocardiography, there was massive tricuspid regurgitation, severe right ventricular (RV) dilation with a diameter (RVD) of 31 mm, a significant reduction in ejection fraction, and abnormal septal motion were noted. Contrast computed tomography of the chest was postponed due to concerns about an allergic reaction to the angiographic drugs and unstable vital signs. An APE was considered. Immediately an emergent cesarean birth was scheduled after a multidisciplinary consultation. Abruptly, the birth-giving woman suffered cardiac arrest with severe hypotension noted just after a live fetus and placenta were delivered. Cardiopulmonary resuscitation (CPR) was started right away and was successful following by an operation without a hitch. After that, she was unwell as before and was immediately transported to the intensive care unit (ICU).
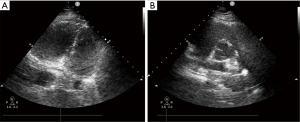
Just arriving at the ICU, a repeat echocardiography showed the same thrombus in the pulmonary artery as the last time (Figure 1), and a left femoral vein thrombosis could be found by lower-limb compression ultrasonography, and arterial blood gas analysis showed refractory metabolic acidosis and hypercapnia. Unfortunately, cardiac arrest occurred again after 30 minutes and was immediately succeed with CPR and endotracheal intubation. But the patient still had persistent severe hypoxia, hypotension, and serious acidosis, requiring high-dose pressor drugs (norepinephrine 0.2 µg/kg/min, epinephrine 0.8 µg/kg/min) to maintain BP. And under pure oxygen conditions, mechanical ventilation could not preserve the oxygenation (SpO2 is less than 90%). The referral was made to proceed with V-V ECMO, 17-French and 23-French cannulae were placed into the right internal jugular vein and femoral vein respectively. Ultrasound confirmed the correct location of the cannulae, and heparin-coated V-V ECMO assistance was commenced with an initial blood flow of 5 L/min and running speed of 3,635 RPM.
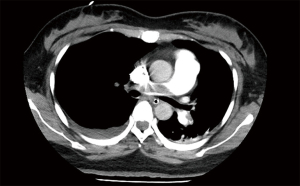
Following one hour, the SpO2 rose to 96%, the BP rose to 100/40 mmHg, the central venous pressure (CVP) decreased from 20 to 6 mmHg. The patient’s haemodynamics and respiratory function improved significantly. The dose of vasopressor was reduced (norepinephrine 0.15 µg/kg/min, epinephrine 0.25 µg/kg/min) with stable circulation. Repeat arterial blood gas analysis showed that the acidosis improved. But vaginal bleeding increased gradually in 1 hour to an estimated 250 mL, and hematocrit (HCT) decreased to 24%, so she was transfused with red blood cells 4U and plasma 800 mL. This is why anticoagulation could not be initiated in the meantime. The next day, with the assistance of the V-V ECMO, computed tomographic pulmonary angiography (CTPA) was conducted successfully, which confirmed the bilateral main pulmonary artery emboli and filling defect with a more apparent right branch (Figure 2), further reinforcing the diagnosis of MPE. At once, bilateral uterine artery embolism was administered priorly with the intention to start anticoagulation and thrombolysis as soon as possible but minimizing the risk of bleeding. Furthermore, the following finished pulmonary angiography, the “gold standard”, also ascertained the diagnosis of PE that there were filling defects in the main pulmonary trunk pulmonary arteries to the right lung, meanwhile, which assisted in clot breakdown with placement of thrombolysis infusion catheters, the blocked pulmonary artery was reopened (Figure 3A,3B). The patient adopted thrombolysis with urokinase at 100,000 IU/Qd first, followed by a progressive dose from 200,000 to 500,000 IU/Bid with the monitoring of lochia rubra amount, besides, combined with systemic sequential heparin therapy at an adjustable dose (2 mL/h originally) to maintain target activated partial thromboplastin time ratio (APTT) was 1.5–2.0 times continuously. The daily serial echocardiography and coagulation function also worked to investigate current thrombus size, cardiac function and bleeding risk, taking more steps to evaluate whether there was a terminal for thrombolysis or ECMO, and practically showed gradual normalization of RV systolic function and pulmonary artery pressures.
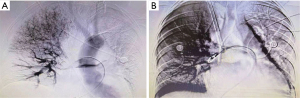
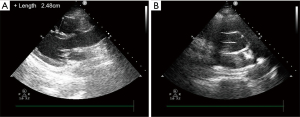
After 5 days of medical perfusion therapy, heparin and urokinase were suspended owing to aberrant APTT (>180 s), followed by vitamin K and protamine to rectify coagulopathy. On the same day, the patient underwent bilateral pulmonary angiography again, which revealed a diminution of thrombus. Regarding apparent improvement in symptoms, heart function or hematal obstruction in echocardiography (Figure 4), the patient was successfully weaned from ECMO and an inferior vena cava filter was embedded for the sake of another mechanical block in the pulmonary artery induced by venous clots from lower limb vessels. Then, she was transferred from the ICU to the obstetric department. Twenty days later, the patient was discharged home with no residual deficit. At 1 month post discharge, the RV size and function had normalized and the bilateral pulmonary artery thrombosis was significantly reduced with better contractibility compared with the previous ones. And the vena cava filter was removed. The patient remained on rivaroxaban therapy with regular check of coagulation function, and she had a thriving, healthy infant according to our regular follow-up. Moreover, she expressed sincere gratitude for the prompt and efficacious therapy. The timeline (Figure 5) shows the major interventions and outcomes.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Oral informed consent for publication of this case report and accompanying images was obtained from the patient or the relatives, and the anonymity is retained.
Discussion
MPE generates a significant increase in pulmonary artery pressure through mechanical obstruction (8), combined with pulmonary artery contraction caused by neurohumoral factors and hypoxia, which will result in RV failure (9), contributes further to systemic hypotension and haemodynamic instability (10). Study has demonstrated that the 90-day mortality for individuals with cardiac arrest and hypotension in MPE is as high as 52%, and many of them die within one hour after symptom onset (11). Therefore, the treatment to relieve embolism or abnormal vasoconstriction, the key point, has to be respected. In light of embolism, the current recommendation suggests perfusion therapy as the first-line therapy. Nevertheless, ECMO is recommended preferentially at organ resuscitation and striving time for clot breakdown for patients with hemodynamic collapse and contraindications to anticoagulation and thrombolysis (8,10).
To select an appropriate ECMO mode mainly rests on the all-sided hemodynamics, pulmonary condition, along with RV function (12). It is now widely acknowledged that V-A ECMO is the optimal choice for MPE, circulatory collapse, or cardiac arrest or for patients in whom high-dose vasoactive drugs still cannot maintain the circulation (13). It can quickly lessen the right heart load (14), ameliorate the right cardiac function, and better tissue blood perfusion and oxygenation afterwards. However, Kmiec L has proposed that there are several indications for V-V ECMO that circulatory stabilization can be supported by high doses of positive vasoactive agents or systemic hemodynamics recover spontaneously after CPR but merged with refractory respiratory depression even if assisted by energetic ventilation such as large ventilation volume and high airway pressures or positive end-expiratory pressure for increased functional residual capacity (12).
Whether there is lessened flow in clogged pulmonary arteries or zones of overflow in unobstructed pulmonary arteries of MPE (15,16), both of which cause ventilation/perfusion mismatch, enlarge the dead space and the inability to excrete CO2, thereby exacerbating serious hypoxemia and acidosis, which may lead to vascular contraction (16). In addition, the ensuing hypercapnia, inflammatory mediators and neurohumoral factors also intensify pulmonary vasoconstriction (9) more on the basis of mechanical obstruction, which worsens RV dilatation (17) and even failure and organ dysfunction (13). Similarly, after V-V ECMO improves the oxygenation and CO2 removal, followed by the lowering of pulmonary arterial vascular resistance, the RV afterload decreases, and RV function improves subsequently. Then, more pulmonary blood flow and adequate left ventricular (LV) filling boost cardiac output and improve BP.
The patient mentioned in this article was young and had no previous history of cardiovascular disease. Moreover, the heart beat again quickly following CPR, and the hemodynamics remained generally stable with the support of pressor agents, which indicated that the hemodynamic condition was acceptable. Hence, V-V ECMO was operational. Aside from that, feasibility and complications should also be taken into consideration. Indwelling axillary or femoral artery cannulas are required for V-A ECMO. However, there is a higher risk of complications, such as necrosis of limbs due to compartment syndrome, compared to V-V ECMO (18). And, vascular complications such as aneurysm and pseudoaneurysm formation caused by direct arterial puncture for cannulation have been reported to occur seven times more frequently in patients with V-A ECMO than in V-V ECMO (19). A single-center analysis showed patients under V-A ECMO were susceptible to severe hemolysis presenting with high free plasma hemoglobin (fHb) over 500 mg/L, which occurred in 4% while it happened in 2% with V-V ECMO (20). Above all, the operation of V-V ECMO is provided with simplicity as well as less risk. Consequently, considering indication and risk comprehensively, V-V ECMO was administered and severe hypoxia was rectified in time for the patient. After that, the pulmonary artery contraction and spasm were alleviated, the RV afterload was reduced, and the circulation improved with more blood flowing from the right to the left heart. Then, through the follow-up anticoagulation and thrombolysis, the patient achieved a better prognosis. To sum up, relieving vasospasm before thrombosis is a pivotal factor for hemodynamic stabilization through V-V ECMO. But, how can we estimate the ratio of mechanical embolism factors to vasospasm factors as accurately as we can and prognosticate whether there will be an efficacious advance in hemodynamics ahead of choosing the appropriate mode of ECMO remain untoward. When we cannot determine the most appropriate pattern, we can make preparations on both fronts: if the patients had suffered cardiac arrest during V-V ECMO it could have been relatively fast to change to V-A ECMO if the patient had been prepared with a small sheath 6Fr in the femoral artery, making cannulation over a guide wire possible in an emergency (21).
Conclusions
In this report, drug and catheter-directed pulmonary artery thrombolysis combined with ECMO is an efficacious consideration. Additionally, V-V ECMO, which acts as a bridge to thrombolysis, is crucial in the treatment of the patient with an initiative to recover circulation followed by CPR but out of better respiratory function under aggressive ventilation even with refractory hypotension.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-128/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-128/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-128/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Oral informed consent for publication of this case report and accompanying images was obtained from the patient or the relatives, and the anonymity is retained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giraud R, Laurencet M, Assouline B, et al. Can VA-ECMO Be Used as an Adequate Treatment in Massive Pulmonary Embolism? J Clin Med 2021;10:3376. [Crossref] [PubMed]
- Igneri LA, Hammer JM. Systemic Thrombolytic Therapy for Massive and Submassive Pulmonary Embolism. J Pharm Pract 2020;33:74-89. [Crossref] [PubMed]
- Hobohm L, Sagoschen I, Habertheuer A, et al. Clinical use and outcome of extracorporeal membrane oxygenation in patients with pulmonary embolism. Resuscitation 2022;170:285-92. [Crossref] [PubMed]
- Mihu MR, Mageka D, Swant LV, et al. Veno-arteriovenous extracorporeal membrane oxygenation-A single center experience. Artif Organs 2021;45:1554-61. [Crossref] [PubMed]
- Assouline B, Assouline-Reinmann M, Giraud R, et al. Management of High-Risk Pulmonary Embolism: What Is the Place of Extracorporeal Membrane Oxygenation? J Clin Med 2022;11:4734. [Crossref] [PubMed]
- Maggio P, Hemmila M, Haft J, et al. Extracorporeal life support for massive pulmonary embolism. J Trauma 2007;62:570-6. [Crossref] [PubMed]
- Faggian G, Onorati F, Chiominto B, et al. Veno-venous extracorporeal membrane oxygenation as a bridge to and support for pulmonary thromboendarterectomy in misdiagnosed chronic thromboembolic pulmonary hypertension. Artif Organs 2011;35:956-60. [Crossref] [PubMed]
- Weinberg A, Tapson VF, Ramzy D. Massive Pulmonary Embolism: Extracorporeal Membrane Oxygenation and Surgical Pulmonary Embolectomy. Semin Respir Crit Care Med 2017;38:66-72. [Crossref] [PubMed]
- Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primers 2018;4:18028. [Crossref] [PubMed]
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543-603. [Crossref] [PubMed]
- Seaton A, Hodgson LE, Creagh-Brown B, et al. The use of veno-venous extracorporeal membrane oxygenation following thrombolysis for massive pulmonary embolism. J Intensive Care Soc 2017;18:342-7. [Crossref] [PubMed]
- Kmiec L, Philipp A, Floerchinger B, et al. Extracorporeal Membrane Oxygenation for Massive Pulmonary Embolism as Bridge to Therapy. ASAIO J 2020;66:146-52. [Crossref] [PubMed]
- Meneveau N, Guillon B, Planquette B, et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases. Eur Heart J 2018;39:4196-204. [Crossref] [PubMed]
- Pineton de Chambrun M, Bréchot N, Combes A. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock: indications, mode of operation, and current evidence. Curr Opin Crit Care 2019;25:397-402. [Crossref] [PubMed]
- Fernandes CJ, Luppino Assad AP, Alves-Jr JL, et al. Pulmonary Embolism and Gas Exchange. Respiration 2019;98:253-62. [Crossref] [PubMed]
- Baram M, Awsare B, Merli G. Pulmonary Embolism in Intensive Care Unit. Crit Care Clin 2020;36:427-35. [Crossref] [PubMed]
- Rali PM, Criner GJ. Submassive Pulmonary Embolism. Am J Respir Crit Care Med 2018;198:588-98. [Crossref] [PubMed]
- Fernandes P, Allen P, Valdis M, et al. Successful use of extracorporeal membrane oxygenation for pulmonary embolism, prolonged cardiac arrest, post-partum: a cannulation dilemma. Perfusion 2015;30:106-10. [Crossref] [PubMed]
- Douraghi-Zadeh D, Logaraj A, Lazoura O, et al. Extracorporeal membrane oxygenation (ECMO): Radiographic appearances, complications and imaging artefacts for radiologists. J Med Imaging Radiat Oncol 2021;65:888-95. [Crossref] [PubMed]
- Appelt H, Philipp A, Mueller T, et al. Factors associated with hemolysis during extracorporeal membrane oxygenation (ECMO)-Comparison of VA- versus VV ECMO. PLoS One 2020;15:e0227793. [Crossref] [PubMed]
- Kjaergaard B, Kristensen JH, Sindby JE, et al. Extracorporeal membrane oxygenation in life-threatening massive pulmonary embolism. Perfusion 2019;34:467-74. [Crossref] [PubMed]
Cite this article as: Wu H, Liu S, Yang R, Li H. Veno-venous or veno-arterial extracorporeal membrane oxygenation support for massive pulmonary embolism: a case report. AME Case Rep 2024;8:79.


