
HyperArc radiotherapy for recurrent synovial sarcoma of infratemporal fossa: a rare case report and review of the literature
Highlight box
Key findings
• Radical concurrent chemoradiotherapy with HyperArc (HA) radiotherapy (RT) and cisplatin for recurrent synovial sarcoma (SS) of infratemporal fossa (ITF).
What is known and what is new?
• SS occurring within the ITF are exceptionally rare, with a bit number of documented cases worldwide. Much of our understanding from limited case reports, with a lack of established clinical guidelines for its management. So, there is a notable postoperative recurrence rate in SS of ITF, and effectively controlling tumor recurrence poses significant difficulties. This recurrent scenario often leads to diminished postoperative survival outcomes for patients.
• We successfully treated a patient with recurrent SS of ITF by radical concurrent chemoradiotherapy with HA RT and cisplatin, resulting in a remarkable 3-year follow-up period devoid of recurrence.
What is the implication, and what should change now?
• We employed a comprehensive treatment strategy involving concurrent chemoradiotherapy to manage recurrent SS of ITF, which was effective in achieving disease control and improving patient outcomes.
Introduction
Background
Synovial sarcoma (SS), constituting the fourth most common soft tissue tumor, presents with an annual incidence of approximately 2.75 cases per million individuals, accounting for 5–10% of all soft tissue tumors, which predominantly affects the extremities and trunk (1). However, SS located specifically within the infratemporal fossa (ITF) represent an exceedingly rare subset (2). Stanbouly et al. (3) counted SS of ITF reported worldwide since 1950 and a mere 14 cases had been documented globally.
Rationale and knowledge gap
The primary treatment approach for SS typically involves surgical intervention followed by adjuvant chemoradiotherapy (4). However, managing SS of ITF presents unique challenges due to the intricate nature of adjacent anatomical structures. ITF communicates with the skull via the foramen ovale and spinous foramen, extends forward to the orbit through the infraorbital fissure, and inwardly connects to the pterygopalatine fossa via the pterygopalatine fissure. These pathways serve as crucial channels for potential tumor spread and invasion (5). Completing radical surgery for SS of ITF proves to be particularly challenging, given the complexity of adjacent organs and structures. Consequently, there is a notable postoperative recurrence rate, and effectively controlling tumor recurrence poses significant difficulties. This recurrent scenario often leads to diminished postoperative survival outcomes for patients (6).
Objective
We present the case of a patient diagnosed with SS of ITF, who underwent two surgical procedures along with multiple rounds of chemotherapy, yet experienced persistent in situ recurrence. We opted to administer concurrent chemoradiotherapy with HyperArc (HA) radiotherapy (RT) and cisplatin. Given the complexity and rarity of the case, we deemed it valuable to share our experience. Through this report, we delve into the clinical and pathological characteristics of this disease, elaborate on the treatment strategy employed, and discuss the prognosis associated with this condition. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-88/rc).
Case presentation
A 35-year-old female with no past history presented with a progressive enlargement of a right facial mass accompanied by numbness and pain in the right face, as well as swelling and pain in the right periorbital region and eye. Magnetic resonance imaging (MRI) (Figure 1) revealed a well-defined mass located within the right ITF. The mass exhibited characteristics of enlargement of ITF, along with compression, displacement, and destruction of the surrounding bone. On T1-weighted image (T1WI), the signal intensity of the mass corresponded to that of the surrounding muscles. Conversely, on T2-weighted image (T2WI), the signal intensity appeared homogeneous and low. Following the administration of contrast medium, mild enhancement of the mass was observed. Following radical surgery, histopathological examination was conducted (Figure 2). Hematoxylin and eosin (HE) staining revealed distinctive features: the tumor cells exhibited a distinctive arrangement, forming ducts or adenoid structures interspersed with multiple papillary or cystic cavities. Morphologically, the tumor cells displayed a spectrum of shapes, ranging from columnar, cuboidal to polygonal, with some cells adopting a spindle-shaped morphology. Notably, these cells featured clear cytoplasm and were organized in a sheet-like configuration. Additionally, epithelioid cells were observed sporadically, arranged in a grid-like pattern. Calcification was also identified within the tumor tissue. Immunohistochemical (IHC) (Figure 3) staining showed that S100 (+), CD34 (+), cytokeratin (CK) (+), beta-catenin (+), smooth muscle actin (SMA) (part+), BCL-2 (part+), CD99 (+), Ki-67 (20%+), p53 (+), epithelial membrane antigen (EMA) (−), CK7 (−), CK19 (−), CK8/18 (−), melanA (−), anaplastic lymphoma kinase (ALK) (−), HMB45 (−), desmin (−), STAT6 (−). It was confirmed to be a fibrous SS of ITF, with a positive margin. The patient was diagnosed with SS of ITF (cG3T2bN0M0, stage IIIA).



One-year post-operation, the patient presented with recurrent symptoms of right facial numbness and swelling. Radiological examination revealed the involvement of the right maxillary sinus by the right infratemporal mass, along with invasion into the medial and lateral pterygoid muscles. Pathological confirmation affirmed in situ recurrence of the malignant tumor. Subsequently, the patient underwent extended resection of the recurrent tumor within the right ITF and maxilla, and the resection margin was still positive. Following surgery, the patient received a four-cycle AI regimen (doxorubicin plus ifosfamide). However, the patient declined further RT due to experiencing severe adverse reactions. One year subsequent to the previous relapse, the patient experienced a second in situ recurrence. The tumor emerged within the right orbit and beneath it, progressively enlarging and causing pain in the right orbit, along with a decline in vision in the right eye. Radiological assessment disclosed the presence of a mass within the right ITF, exhibiting invasion into adjacent structures. Consequently, the patient’s diagnosis was revised to recurrent SS of the right ITF (rG3T2bN0M0, stage IIIA).
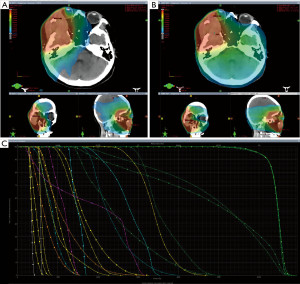
The patient presented with an Eastern Cooperative Oncology Group (ECOG) performance status score of 1. We believed that concurrent chemoradiotherapy could serve as an effective therapeutic approach in this case. Given the tumor’s involvement of the orbit and the paramount importance of preserving the patient’s vision during RT, we devised a gradient-fractionated RT plan (Figures 4,5). Planning target volume (PTV): 5,200 cGy/26 Fx, after a reduction field addition of 1,200 cGy/6 Fx, a further reduction field addition of 600 cGy/3 Fx. HA RT was chosen because it can reduce the dose of organs at risk (OARs) and protect the cornea, optic nerve, and other vital organs. In addition, we chose single-agent concurrent chemoradiotherapy with cisplatin.

The patient exhibited no significant adverse reactions to RT during treatment, and her visual acuity returned to normal. Remarkably, 1-month post-treatment, a substantial reduction in tumor size was observed (Figure 6). Subsequent follow-up examinations were conducted every 3 months thereafter. Six months following completion of RT, the patient underwent reexamination, revealing complete regression of the tumor. Evaluation indicated that the tumor had reached complete response (CR). Encouragingly, over the course of 3 years, there has been no evidence of tumor recurrence, signifying the effectiveness of the treatment and the limited occurrence of adverse reactions. The timeline of the treatment of the patient is shown in Figure 7.


Patient perspective
After the onset of the disease, I went through two painful procedures of surgery and chemotherapy, but still failed to prevent its recurrence. However, after the RT, my symptoms completely disappeared, my vision recovered, and I was able to participate in daily activities, including housework, such as cleaning. At the moment, my illness is completely under control and I just need regular follow-ups.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
SS, represents a subset of soft tissue sarcomas, constituting approximately 5–10% of all cases in this category. SS typically arises within the deep soft tissues of the extremities. However, instances of SS occurring within the ITF are exceptionally rare (7). SS typically manifests as a deep-seated, painless mass characterized by ill-defined borders and limited mobility in its early stages. As the tumor progresses, pain may ensue, accompanied by symptoms indicative of compression of surrounding tissues (8). The duration of symptoms can vary widely, ranging from 2 months to 4 years (9). Patients younger than 20 years old, presenting with well-differentiated tumors localized in the extremities, typically have a favorable prognosis. However, prognosis significantly declines for patients with unfavorable factors such as metastases, poorly differentiated tumors, and tumors larger than 5 cm, particularly when located in the head and neck region. The 5-year survival rate for such patients is less than 30% (10).
SS commonly presents as a well-circumscribed, capsular mass exhibiting either homogeneous or heterogeneous enhancement on imaging studies. In some instances, it may manifest as a cystic mass, often attributed to intrafocal hemorrhage or necrosis (11-13). The diagnosis of SS relies on histopathological examination, which typically reveals a composition of epithelial cells resembling cancer cells and fibrosarcoma-like spindle cells. Based on the differentiation and composition of these two cell types, SS can be classified into four distinct subtypes: biphasic, monophasic spindle cell, monophasic epithelial, and poorly differentiated (round cell) types (14). The results of IHC and special staining serve as valuable adjuncts in the diagnosis of SS. IHC markers such as EMA, CKpan, CK7, CK19, vimentin, calponin, BCL-2, CD99, TLE1, and INI1 are useful (15). Additionally, chromosomal translocation t(x;18)(p11.2;q11.2), resulting in fusion of SS18 (SYT) with SSX1 or SSX2 on the X chromosome, serves as the gold standard for diagnosing SS (16,17). Unfortunately, due to financial constraints, the patient did not undergo fluorescence in situ hybridization (FISH) testing.
The primary treatment modality for SS is surgical intervention, typically involving extensive or radical resection aimed at achieving negative margins. Adjuvant chemoradiotherapy is often administered following surgery (18). Primary SS exhibits greater sensitivity to chemotherapy compared to other soft tissue sarcomas. A phase 3 randomized clinical trial by Gronchi et al. (19) demonstrated that adjuvant chemoradiotherapy with a high-dose AI regimen improves outcomes in patients with high-risk SS. However, it is important to note that the response to chemotherapy may diminish in cases of metastasis or recurrence of SS (20). In this particular case, the patient underwent four cycles of AI chemotherapy following the second radical resection. However, the recurrence occurred despite this treatment regimen, highlighting the limited efficacy of doxorubicin chemotherapy for recurrent SS. There have been hopes that targeted drugs, particularly those theoretically combinable with doxorubicin, could offer promising outcomes. Olaratumab, in particular, showed extraordinary success in phase II trials. However, disappointing results from phase III trials failed to replicate the earlier promising findings, leading to its non-approval (21).
RT remains a crucial approach for achieving local control and preventing recurrence in the management of SS. A retrospective study (22) conducted at a single institution demonstrated that patients with SS who underwent surgery followed by postoperative RT achieved impressive local control rates (LCRs) of 90% and 88% at 5 and 10 years, respectively. Furthermore, Gingrich et al. (23) found that RT can significantly enhance the 3-year survival rates of patients with high-grade SS by as much as 10%. In this case, we opted for concurrent chemoradiotherapy due to the patient’s recurrence following two radical surgeries. Another extensive resection posed a risk of damaging peripheral organs such as the eyes and appendages, potentially leading to irreparable consequences for the patient’s quality of life. RT, on the other hand, demonstrated a significant advantage in protecting these peripheral organs. Given the complexity of structures adjacent to the ITF, achieving an ideal negative resection margin through surgery alone is often challenging. Consequently, adjuvant RT plays a crucial role in preventing recurrence in such patients. It’s noteworthy that the patient did not receive RT after the previous surgery, which could be a significant contributing factor to the recurrence. Indeed, previous reports have commonly favored palliative chemotherapy for the management of recurrent SS of ITF, irrespective of prior RT (24). The efficacy of radical RT in patients who have not received timely adjuvant RT remains uncertain. This poses a significant challenge in determining the optimal treatment regimen for such cases.
In head and neck RT, the routine selection is volumetric-modulated arc therapy (VMAT) techniques (25). Indeed, in this case, the tumor’s invasion of the orbit and close proximity to the eyeball necessitated a higher level of target conformability and dose drop rate in developing a graded fractionated RT plan. The complexity of the tumor’s location and its proximity to critical structures such as the eyeball posed significant challenges in ensuring effective treatment while minimizing damage to nearby OARs. HA, provided by the Varian TrueBeam accelerator, represents a novel non-coplanar multi-arc technique that has emerged as a highly effective option for treating intracranial metastases. Numerous studies (26-28) have demonstrated its ability to significantly enhance target uniformity, achieve steep out-of-target dose gradients, reduce doses to OARs, and effectively protect critical structures such as the lens, retina, and optic nerve. The comparison of RT plans for HA and VMAT revealed distinct differences in spatial dose distributions, as illustrated in Figure 5. The isodose lines for the HA plan demonstrated superior closure compared to VMAT. The calculations indicated that HA outperformed VMAT in terms of conformity index (CI), gradient index (GI), and homogeneity index (HI) (Table 1). Additionally, HA demonstrated lower doses to OARs (Table 2), offering superior protection for critical structures such as the cornea, optic nerve, and brainstem. This reduction in radiation exposure to OARs effectively minimizes the risk of adverse effects including vision loss, blindness, and corneal ulceration associated with RT. We believe that the implementation of HA technology has played a pivotal role in mitigating the occurrence of adverse reactions during the RT course. In fact, the entire RT process proceeded smoothly, with only mild side effects reported, and without side effects exceeding grade 2 severity. Moreover, we are pleased to report that the patient’s vision was successfully restored following the completion of RT. We retrieved the relevant case reports of SS in ITF. Patients who receive preoperative or postoperative RT usually have a poor prognosis, with recurrent or distant metastases occurring between 4 and 14 months. The use of intensity-modulated RT (IMRT) or VMAT in these cases, usually without concurrent chemotherapy, is in sharp contrast to our case (29,30).
Table 1
| RT | RTOG CI | Paddick CI | HI | GI |
|---|---|---|---|---|
| HA | 0.93 | 0.87 | 1.14 | 2.03 |
| VMAT | 1.01 | 0.83 | 1.19 | 2.65 |
RTOG, Radiation Therapy Oncology Group; CI, conformity index; HI, homogeneity index; GI, gradient index; VMAT, volumetric-modulated arc therapy; HA, HyperArc.
Table 2
| RT | Chiasm Dmax (cGy) | Brainstem Dmax (cGy) | Lens (cGy) | Cornea (cGy) | Lacrimal gland (cGy) | Retina (contralateral) (cGy) | Optic nerve (contralateral) (cGy) |
|---|---|---|---|---|---|---|---|
| VMAT | 4,878.2 | 4,723.2 | 3,054.3 | 5,713.7 | 6,813.7 | 2,619.7 | 3,544.3 |
| HA | 5,097.3 | 3,271.2 | 2,935.2 | 5,562.3 | 6,685.6 | 1,573.3 | 1,703.6 |
OAR, organ at risk; RT, radiotherapy; Dmax, maximum dose; VMAT, volumetric-modulated arc therapy; HA, HyperArc.
Upon reviewing our case, it is evident that our patient presents a rare and complex manifestation of SS of ITF. Despite undergoing two radical surgeries and multiple cycles of chemotherapy, the disease recurred, underscoring its aggressive nature and challenging management. In light of this, we opted for concurrent chemoradiotherapy, which hold promise for achieving effective control of local recurrence. Cisplatin was chosen as the concurrent chemoradiotherapy agent due to its known ability to enhance the effectiveness of RT. Our treatment strategy has demonstrated notable feasibility and efficacy in managing recurrent SS of ITF. Given the limited previous reports on treatment strategies for this condition, our approach of radical concurrent chemoradiotherapy represents a valuable contribution to the field. The success of our strategy offers valuable insights and guidance for clinicians facing similar challenges in clinical practice.
Conclusions
SS of ITF presents as a rare and challenging condition, particularly in cases of recurrence following multiple surgeries and chemotherapy. With no clear optimal treatment strategy established for its management, there exists a significant clinical need for effective therapeutic approaches. In this case, we employed a comprehensive treatment strategy involving concurrent chemoradiotherapy based on HA. Our findings demonstrate that this approach was effective in achieving disease control and improving patient outcomes, and compared with VMAT, HA provides a better survival benefit.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-88/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-88/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-88/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. [Crossref] [PubMed]
- Harb WJ, Luna MA, Patel SR, et al. Survival in patients with synovial sarcoma of the head and neck: association with tumor location, size, and extension. Head Neck 2007;29:731-40. [Crossref] [PubMed]
- Stanbouly D, Litman E, Lee KC, et al. Synovial sarcoma of the head & neck: A review of reported cases in the literature. J Stomatol Oral Maxillofac Surg 2021;122:505-10. [Crossref] [PubMed]
- von Mehren M, Kane JM, Agulnik M, et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:815-33. [Crossref] [PubMed]
- Kim SM, Paek SH, Lee JH. Infratemporal fossa approach: the modified zygomatico-transmandibular approach. Maxillofac Plast Reconstr Surg 2019;41:3. [Crossref] [PubMed]
- Bin-Alamer O, Bhenderu LS, Palmisciano P, et al. Tumors Involving the Infratemporal Fossa: A Systematic Review of Clinical Characteristics and Treatment Outcomes. Cancers (Basel) 2022;14:5420. [Crossref] [PubMed]
- Gazendam AM, Popovic S, Munir S, et al. Synovial Sarcoma: A Clinical Review. Curr Oncol 2021;28:1909-20. [Crossref] [PubMed]
- Thway K, Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol 2014;18:369-80. [Crossref] [PubMed]
- Xia S, Chen X, Hu Y, et al. Biphasic synovial Sarcoma with extensive calcification in the temporomandibular joint region: A rare case report and literature review. J Stomatol Oral Maxillofac Surg 2020;121:592-8. [Crossref] [PubMed]
- Vlenterie M, Litière S, Rizzo E, et al. Outcome of chemotherapy in advanced synovial sarcoma patients: Review of 15 clinical trials from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group; setting a new landmark for studies in this entity. Eur J Cancer 2016;58:62-72. [Crossref] [PubMed]
- Sedaghat M, Sedaghat S. Primary synovial sarcoma on MRI - a case series and review of the literature. Pol J Radiol 2023;88:e325-30. [Crossref] [PubMed]
- Sedaghat S, Schmitz F, Meschede J, et al. Systematic analysis of post-treatment soft-tissue edema and seroma on MRI in 177 sarcoma patients. Surg Oncol 2020;35:218-23. [Crossref] [PubMed]
- Sedaghat S, Sedaghat M, Meschede J, et al. Diagnostic value of MRI for detecting recurrent soft-tissue sarcoma in a long-term analysis at a multidisciplinary sarcoma center. BMC Cancer 2021;21:398. [Crossref] [PubMed]
- Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv Anat Pathol 2021;28:44-58. [Crossref] [PubMed]
- Vlenterie M, Jones RL, van der Graaf WT. Synovial sarcoma diagnosis and management in the era of targeted therapies. Curr Opin Oncol 2015;27:316-22. [Crossref] [PubMed]
- Baranov E, McBride MJ, Bellizzi AM, et al. A Novel SS18-SSX Fusion-specific Antibody for the Diagnosis of Synovial Sarcoma. Am J Surg Pathol 2020;44:922-33. [Crossref] [PubMed]
- Fiore M, Sambri A, Spinnato P, et al. The Biology of Synovial Sarcoma: State-of-the-Art and Future Perspectives. Curr Treat Options Oncol 2021;22:109. [Crossref] [PubMed]
- Vining CC, Sinnamon AJ, Ecker BL, et al. Adjuvant chemotherapy in resectable synovial sarcoma. J Surg Oncol 2017;116:550-8. [Crossref] [PubMed]
- Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812-22. [Crossref] [PubMed]
- Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 2012;13:1045-54. [Crossref] [PubMed]
- Tap WD, Wagner AJ, Papai Z, et al. ANNOUNCE: A randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox)+ olaratumab versus dox+ PBO in patients (pts) with advanced soft tissue sarcomas (STS). J Clin Oncol 2019;37:LBA3. [Crossref]
- Naing KW, Monjazeb AM, Li CS, et al. Perioperative radiotherapy is associated with improved survival among patients with synovial sarcoma: A SEER analysis. J Surg Oncol 2015;111:158-64. [Crossref] [PubMed]
- Gingrich AA, Marrufo AS, Liu Y, et al. Radiotherapy is Associated With Improved Survival in Patients With Synovial Sarcoma Undergoing Surgery: A National Cancer Database Analysis. J Surg Res 2020;255:378-87. [Crossref] [PubMed]
- Weiss MC, Van Tine BA. Relapsed Synovial Sarcoma: Treatment Options. Curr Treat Options Oncol 2023;24:229-39. [Crossref] [PubMed]
- Wolff HA, Wagner DM, Christiansen H, et al. Single fraction radiosurgery using Rapid Arc for treatment of intracranial targets. Radiat Oncol 2010;5:77. [Crossref] [PubMed]
- Ohira S, Sagawa T, Ueda Y, et al. Effect of collimator angle on HyperArc stereotactic radiosurgery planning for single and multiple brain metastases. Med Dosim 2020;45:85-91. [Crossref] [PubMed]
- Ruggieri R, Naccarato S, Mazzola R, et al. Linac-based radiosurgery for multiple brain metastases: Comparison between two mono-isocenter techniques with multiple non-coplanar arcs. Radiother Oncol 2019;132:70-8. [Crossref] [PubMed]
- Vergalasova I, Liu H, Alonso-Basanta M, et al. Multi-Institutional Dosimetric Evaluation of Modern Day Stereotactic Radiosurgery (SRS) Treatment Options for Multiple Brain Metastases. Front Oncol 2019;9:483. [Crossref] [PubMed]
- Nomura F, Kishimoto S. Synovial sarcoma of the temporomandibular joint and infratemporal fossa. Auris Nasus Larynx 2014;41:572-5. [Crossref] [PubMed]
- Tamarit Conejeros JM, Estrems Navas P, Estellés Ferriol E, et al. Synovial sarcoma of the infratemporal fossa. Acta Otorrinolaringol Esp 2010;61:389-91. [Crossref] [PubMed]
Cite this article as: Li W, Lou F, Zhai L, Piao M, Zhu Y, Liu S, Li K, Chen L, Wang H. HyperArc radiotherapy for recurrent synovial sarcoma of infratemporal fossa: a rare case report and review of the literature. AME Case Rep 2024;8:104.


