
A rare case of symptomatic congenital lobar emphysema in an adolescent female who underwent expectant management of a congenital pulmonary malformation: a case report
Highlight box
Key findings
• A 17-year-old female who underwent expectant management for congenital lobar emphysema (CLE) in infancy presented with new-onset pre-syncope, tachycardia, and dyspnea. The patient underwent an uncomplicated thoracotomy with left upper lobectomy and experienced complete resolution of symptoms.
What is known and what is new?
• Conservative management of asymptomatic CLE is accepted, though it is unknown how many patients go on to require definitive surgical treatment.
• A description of a rare case of CLE in a female presenting with pre-syncope as a chief complaint in adolescence is provided.
What is the implication, and what should change now?
• This report highlights that expectant management may be successful in patients with asymptomatic congenital pulmonary malformations, though clinicians should recognize that symptoms may be atypical in adolescence and overinflation of the diseased lobe should inform the surgical approach.
Introduction
Background
Congenital lung malformations (CLMs) are increasingly being identified on antenatal ultrasound and occur at an incidence of 1 in 2,500 to 1 in 8,000 live births (1). Congenital pulmonary airway malformations (CPAMs), previously known as congenital cystic adenomatoid malformation (CCAM), are the most common (30–40%) of these lesions (1,2). CPAMs are characterized by an abnormal airway pattern occurring during lung branching morphogenesis and can possibly lead to cystic and/or adenomatous pulmonary areas (2). CPAM has been categorized into five types by Stocker, based on the size of cysts and anatomic segment of injury along the tracheobronchial tree from the proximal bronchus to distal acinus: Incomplete lung development or hypoplasia (type 0), large cysts measuring up to 7 cm (type 1), cysts less than 2 cm (type 2), cysts measuring up to 0.5 cm (type 3), and distal acinar cysts (type 4) (3).
The differential diagnosis for CPAMs includes bronchopulmonary sequestration, bronchogenic cysts, and congenital lobar emphysema (CLE) (2). The latter are particularly challenging to differentiate from CPAMs. CLEs represent approximately 10% of CLMs and are characterized by overinflation of the lung. They occur at an incidence of 1 in 20,000 to 1 in 30,000 live births (1,4). The most common location of a CLE is the left upper lobe (4). Etiology is often idiopathic; hypoplasia, dysplasia, and the absence of primary or secondary bronchial cartilage may be observed (4,5). Air trapping leads to hyperinflation of the affected lobe and overdistention of alveoli. Damage to the alveoli makes them permanently ineffective for gas exchange.
Approximately half of patients with CLE present at birth, while the other half develop symptoms in the first 6 months of life (4). Patients with CLE often present with cough, dyspnea, cyanosis, and difficulty feeding (4). Patients may also develop recurrent respiratory infections (4). In rare cases, patients are mistakenly diagnosed with pneumonia and pneumothorax (6). CLEs are accompanied by cardiac malformations in 14–20% of cases (4).
Rationale and knowledge gap
There is consensus that symptomatic patients with CPAM or CLE should undergo operative management, whereas the management of asymptomatic patients is widely debated. Both surgical resection and observation have been proposed, with limited literature supporting either option (4,7). Arguments in favor of resection include the possibility of delayed surgical intervention being complicated by recurrent infection or acute respiratory distress, and the association of malignancy (e.g., pleuropulmonary blastoma) with CPAMs (8,9). In contrast, conservative follow-up is supported by the fact that the majority of lesions remain asymptomatic and some even regress spontaneously (8,9). Currently, for asymptomatic patients who undergo observation, no surveillance guidelines have been defined and appear to be based on clinician preference (8). The exact timing and modalities of neonatal imaging for asymptomatic lesions remain under debate, however, computed tomography (CT) has proposed within the first 6 months of life (2). Antenatal and postnatal radiological features are unable to distinguish lesions that may resolve from those that progress or become symptomatic. At present, there is limited knowledge on how many patients become symptomatic later in life, requiring definitive surgical treatment, thus limiting the development of treatment guidelines. Due to the rarity of these cases, optimal surgical management remains unclear.
Objective
The aim of this work was to present a rare case of symptomatic CLE in an adolescent female previously thought to have a CPAM, highlighting perioperative considerations for the surgical team that may benefit this rare group of patients. This case also raises the question as to whether lifelong surveillance of pulmonary lesions detected in infancy would be useful. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-28/rc).
Case presentation
A 17-year-old female was referred to Thoracic Surgery for a one-year history of pre-syncopal episodes, intermittent tachycardia detected on a smartwatch, and dyspnea on exertion, in the context of known left upper lobe CPAM. On history, she had been diagnosed with a CPAM at birth. CT thorax at 3 months of age demonstrated cystic changes to the left lower lobe, which was felt to be suggestive of type II CPAM (see Figure 1). The decision was made to proceed with observation, and at one year of age, a CT was repeated. This showed a large geographic region within the left lung with hypoattenuating pulmonary parenchyma. Based on the vasculature, this was felt to be involving the left upper lobe. Note was made of hyperexpansion secondary to air trapping, consistent with CLE, though bronchial atresia remained in the differential diagnosis (see Figure 2). This lesion was also monitored, and a chest X-ray (CXR) at age 2 years was within normal limits. Following this, she did not undergo any regular surveillance, and remained asymptomatic until age 17 years.

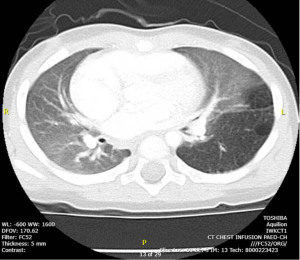
On clinical assessment, the patient appeared generally well and remained hemodynamically stable on room air. A CT thorax demonstrated hyperinflation of the left lung causing displacement of the mediastinum to the right, felt to represent sequelae of the previously diagnosed CPAM (see Figure 3). An echocardiogram was performed and found to be within normal limits.

The decision was made to proceed with left upper lobectomy, for treatment of the patient’s symptoms. Intraoperatively, bronchoscopy revealed that the airways were narrow, though felt to be within normal limits. A left posterolateral thoracotomy was performed. Upon entry to the chest, there were multiple adhesions between the upper lobe and chest wall. The upper lobe appeared to occupy approximately 80% of the hemithorax. There were notable dense adhesions at the hilum. Once the lobe was freed and removed from the chest, hilar nodes were sampled. A chest tube was inserted, and the left lower lobe was allowed to re-inflate. Initially, it volvulized, therefore was allowed to re-collapse before being successfully re-inflated. The parenchyma fully re-expanded and appeared healthy. Over the course of the surgery, the patient required a fraction of inspired oxygen (FiO2) of 21% while on single lung ventilation, suggesting the left lung had not been significantly contributing to oxygenation.
Post-operatively, the patient did well. Her recovery was unremarkable and largely focused on pain control. CXRs showed a persistent pneumothorax, both before and after removal of the chest tube, which was expected, given the small residual left lower lobe noted intra-operatively. This space was managed expectantly. She was discharged home 5 days after surgery. In subsequent follow-up, she had developed a cough but was otherwise fully recovered. A repeat CXR, at 6 weeks, showed resolution of the left pneumothorax.
Pathology of the left upper lobe of lung revealed congenital lobar hyperinflation. Patchy emphysema, bronchiectasis, and mucostasis were noted in the specimen. No other abnormalities were present. Biopsies of the hilar lymph nodes were without significant histopathologic abnormalities.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images which are also declassified from patient identifiers. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
In this report, we present the rare case of a 17-year-old female who developed pre-syncope, tachycardia, and dyspnea on exertion, secondary to a CLE that was previously thought to have resolved in childhood. She subsequently underwent a left upper lobectomy via thoracostomy. Dense hilar adhesions were noted, though the procedure was uncomplicated. Post-operatively, she had a residual pneumothorax, which resolved over time. The patient’s symptoms of pre-syncope, tachycardia and dyspnea fully resolved following surgical intervention.
Strengths and limitations
This report has several strengths. To the best of our knowledge, this is the first time pre-syncope and tachycardia have been reported as the chief complaint for CLE in an adolescent female. The patient’s entire peri-operative course has been described, and a positive outcome resulting from important operative considerations is provided. This work is nevertheless limited by its observational nature as a case report, restricting generalizability to other patients presenting with CLE in adolescence or adulthood.
Comparison with similar researches
Few patients with CLE present in adolescence or adulthood, and even when diagnosed, surgical resection is very rarely required. When surgery is pursued, the indication is typically for infection, dyspnea, or spontaneous pneumothorax. This has been exemplified in the literature.
Santra and colleagues described the rare case of a 15-year-old male who presented with a 5-month history of exertional breathlessness and was subsequently diagnosed with CLE, based on radiological and bronchoscopic findings (10). Despite identification of a hyperinflated right middle and lower lobe with collapsed right upper lobe, leftward mediastinal shift, and left lung atelectasis, the decision was made to pursue conservative management, as the patient’s symptoms were mild (10). Similarly, there have been reports of an 18-year-old male and a 27-year-old male with newly diagnosed CLE of the left upper lobe, following presentation with an upper respiratory tract infection (11,12). In both cases, the decision was made to pursue non-operative management as there was minimal mediastinal shift on imaging and the patients had no respiratory symptoms, once the acute infection resolved (11,12). In another publication, a 20-year-old female presented acutely to the emergency department with dyspnea and pleuritic pain following a commercial flight, and was found to have a left sided CLE on imaging (13). Following resolution of her symptoms, conservative management was recommended (13). In all of these reports, it is described that the patients continued to have regular follow-up in clinic, though details of ongoing surveillance were not provided.
Indications for surgery in adult patients diagnosed with CLE have included hemoptysis (14), pneumothorax (15,16), and severe dyspnea (17,18). In most cases, video-assisted thoracoscopic surgery (VATS) has been adopted with success (14-16,18).
Explanations of findings
This presentation of CLE described herein is unusual, as CLE is typically diagnosed in infancy, more commonly affects males than females (ratio 3:1) (4), and rarely, if ever, do patients present with a chief complaint of presyncope and tachycardia. When diagnosed in adults, the presenting symptom is usually respiratory distress caused by ventilation-perfusion mismatch (15). This mismatch results from atelectasis of the ipsilateral or contralateral lung and mediastinal shift compression, caused by hyperinflation of one or more emphysematous lobes (15). In this case, the patient noted tachycardia, detected on her smartwatch, which prompted a cardiac work-up. It is important for clinicians to recognize that congenital cardiac anomalies, most commonly ventricular septal defects, may be associated with CLEs (4).
The accepted treatment of CLE is resection of the affected lobe or lobes, which is usually curative (4,18,19). Early surgery is recommended to prevent the complications of lobar over distension, though, increasingly, patients who are asymptomatic or who exhibit mild symptoms may be treated conservatively. VATS seems to be an emerging approach for resection of CLE (19). In this case, an open thoracotomy was used rather than a VATS approach because lung isolation was expected to be difficult secondary to air trapping in the left upper lobe. Dense adhesions around the hilum of the lung and hilar lymphadenopathy secondary to chronic inflammation made the hilar dissection more difficult. Although a VATS approach can be considered, even in centers with a high volume of minimally invasive pulmonary surgery, the operating team should be prepared for an open approach to improve exposure during the hilar dissection.
Implications and actions needed
The current literature demonstrates that CLE is a rare diagnosis in adolescence or adulthood, with only a few case reports published on the topic. To the best of our knowledge, earlier diagnosis of lung pathology in these patients was absent, unlike for our patient, where CPAM was diagnosed in infancy. With increasing antenatal surveillance, it may be postulated that a greater number of asymptomatic congenital pulmonary lesions are being detected and surveilled beyond infancy (7), as was the case described herein. Nevertheless, there is an ongoing knowledge gap on how (i.e., the frequency and duration) surveillance should be conducted, and when these patients require surgery. Various survey studies have shown that management of asymptomatic CPAMs is largely surgeon dependent. In a 2018 survey of members of the European Pediatric Surgeon’s Association, 75% (n=136) of respondents indicated that they operated on asymptomatic CPAMs, usually between 6 and 12 months of age (20). Similarly, in a survey of consultant members of the British Association of Pediatric Surgeons, 21% (n=7) and 56% (n=19) respondents reported that they always or sometimes resect asymptomatic lesions, respectively (21), whereas a 2018 Canadian survey showed that 2%, 35% and 37% of pediatric surgeons always, almost always, or sometimes offer observational management to asymptomatic patients, respectively (22). A systematic review by the American Pediatric Surgery Association, found limited evidence regarding when patients with asymptomatic CPAM should undergo surgery, although data suggests early surgery (i.e., at less than 6 months of age) is safe and allows time for compensatory lung growth (8). With regards to CLE, due to its rarity, evidence for observational management is more sparse. There is a clear need for prospective longitudinal studies to assist in guideline recommendations for the treatment of CLMs.
The case described herein supports that surveillance to the point of lesion resolution on CXR may be acceptable. Our patient avoided any undo harm from repeated imaging in childhood and a diagnosis was successfully made once she developed symptoms at age 17 years. Clinicians, however, must be mindful that symptoms experienced in adolescents/adults with CLE may include pneumothorax, hemothorax, and as this case highlights, presyncope. There should be careful consideration of the operative approach, due to overdistention of the diseased lobe and potential adhesions, secondary to chronicity of the disease.
Conclusions
In summary, we presented the case of a 17-year-old female diagnosed with CLE, in the context of known congenital pulmonary malformation. Her presenting symptoms were pre-syncope, tachycardia, and dyspnea. Though rare, clinicians should be aware that patients managed expectantly for CLE in infancy may require operative management later in life. Prior to surgery, cardiac workup should be completed and operative approach (VATS vs. lobectomy) carefully selected.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-28/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-28/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-28/coif). D.G.F. reports that he has been the facilitator of a physician advisory meeting for AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images which are also declassified from patient identifiers. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zobel M, Gologorsky R, Lee H, et al. Congenital lung lesions. Semin Pediatr Surg 2019;28:150821. [Crossref] [PubMed]
- Leblanc C, Baron M, Desselas E, et al. Congenital pulmonary airway malformations: state-of-the-art review for pediatrician's use. Eur J Pediatr 2017;176:1559-71. [Crossref] [PubMed]
- Dehner LP, Schultz KAP, Hill DA. Congenital Pulmonary Airway Malformations With a Reconsideration and Current Perspective on the Stocker Classification. Pediatr Dev Pathol 2023;26:241-9. [Crossref] [PubMed]
- Demir OF, Hangul M, Kose M. Congenital lobar emphysema: diagnosis and treatment options. Int J Chron Obstruct Pulmon Dis 2019;14:921-8. [Crossref] [PubMed]
- Tuğcu GD, Polat SE, Soydaş SSA, et al. Surgery versus conservative management in congenital lobar emphysema: follow up and indicators for surgery. Pediatr Surg Int 2022;38:559-68. [Crossref] [PubMed]
- Ibrahim R, Ali S, Darwish B. Congenital lobar emphysema case report: A frequently misdiagnosed disease. Ann Med Surg (Lond) 2022;78:103766. [Crossref] [PubMed]
- Kunisaki SM. Narrative review of congenital lung lesions. Transl Pediatr 2021;10:1418-31. [Crossref] [PubMed]
- Downard CD, Calkins CM, Williams RF, et al. Treatment of congenital pulmonary airway malformations: a systematic review from the APSA outcomes and evidence based practice committee. Pediatr Surg Int 2017;33:939-53. [Crossref] [PubMed]
- Kersten CM, Hermelijn SM, Mullassery D, et al. The Management of Asymptomatic Congenital Pulmonary Airway Malformation: Results of a European Delphi Survey. Children (Basel) 2022;9:1153. [Crossref] [PubMed]
- Santra A, Dutta P, Manjhi R, et al. Congenital lobar emphysema presenting at late childhood: A rare case report. Lung India 2014;31:302-4. [Crossref] [PubMed]
- Khalid M, Saleemi S, Khan B. Congenital lobar emphysema in adult: A rare case report. Respiratory Medicine CME 2010;3:150-2. [Crossref]
- Gulsen A. Congenital segmental emphysema in an adult patient. Niger J Clin Pract 2019;22:1163-5. [Crossref] [PubMed]
- Dâmaso S, Carreira NR, Gonçalves C, et al. Congenital Lobar Emphysema in Early Adulthood. Cureus 2021;13:e12590. [PubMed]
- McNamee MM, Harvell RT, Benton MH, et al. Congenital Lobar Emphysema Developing Massive Hemoptysis in Adulthood Treated by Thoracoscopic Lobectomy. Am Surg 2023;89:3284-5. [Crossref] [PubMed]
- Asnake ZT, Salabei JK, Pierce J, et al. An atypical case of congenital lobar emphysema in an adult, non-smoker patient presenting with pneumothorax. Respir Med Case Rep 2021;34:101435. [Crossref] [PubMed]
- King N, Ramesh SS, Essandoh M, et al. Near Complete Obliteration of the Left Hemithorax by Congenital Lobar Emphysema in an Adult. Ann Thorac Surg 2017;104:e367-9. [Crossref] [PubMed]
- Critchley PS, Forrester-Wood CP, Ridley PD. Adult congenital lobar emphysema in pregnancy. Thorax 1995;50:909-10. [Crossref] [PubMed]
- Ryuko T, Yamamoto H, Sugimoto S, et al. Completely Video-assisted Thoracoscopic Lobectomy for Congenital Lobar Emphysema in a Young Adult. Acta Med Okayama 2022;76:89-92. [PubMed]
- Bawazir OA. Congenital lobar emphysema: Thoracotomy versus minimally invasive surgery. Ann Thorac Med 2020;15:21-5. [Crossref] [PubMed]
- Morini F, Zani A, Conforti A, et al. Current Management of Congenital Pulmonary Airway Malformations: A "European Pediatric Surgeons' Association" Survey. Eur J Pediatr Surg 2018;28:1-5. Erratum in: Eur J Pediatr Surg 2018;28:e1. [Crossref] [PubMed]
- Peters RT, Burge DM, Marven SS. Congenital lung malformations: an ongoing controversy. Ann R Coll Surg Engl 2013;95:144-7. [Crossref] [PubMed]
- Robinson A, Romao R, Mills J, et al. Decision-Making Criteria for Observational Management of Congenital Pulmonary Airway Malformations (CPAMs). J Pediatr Surg 2018;53:1006-9. [Crossref] [PubMed]
Cite this article as: Robinson AM, French DG, Davies D. A rare case of symptomatic congenital lobar emphysema in an adolescent female who underwent expectant management of a congenital pulmonary malformation: a case report. AME Case Rep 2024;8:98.


