
A case report of the diagnosis and treatment of immune checkpoint inhibitor-related encephalitis induced by camrelizumab
Highlight box
Key findings
• We presented a case report of the diagnosis and treatment of camrelizumab-related encephalitis.
What is known and what is new?
• Immune checkpoint inhibitor-related side effects are not common.
• The present study presented a case report of the diagnosis, treatment and outcome of camrelizumab-induced encephalitis.
What is the implication, and what should change now?
• This study implied the efficacy and safety of corticoids in the treatment of camrelizumab-related adverse effects.
• Corticoids should be first considered for the treatment of programmed cell death 1-related adverse effects.
Introduction
T cells play a critical role in the initiation and development of various cancers (1). During infection and inflammation, antigens are presented as major histocompatibility complex (MHC) to naive T cells by antigen-presenting cells (APCs), heterogeneous immune cells including macrophages, dendritic cells and B cells (2). These MHC could lead to T cell activation through interaction with T cell receptor/cluster of differentiation 3 (TCR/CD3) complex (3). The TCR/CD3 complex mainly includes two TCR chains (αβ or γδ heterodimer) and six chains of CD3 dimers (CD3ϵγ, CD3ϵδ and CD3ζζ) (4). Functionally, the TCR co-receptors CD4 and CD8 help TCR/CD3 complex to recognize and select antigens on MHC molecules (CD4, MHC class II; CD8, MHC class I) (2). Besides, T cell activation requires TCR costimulatory receptor CD28, which is involved in many processes such as differentiation, cytokines production and proliferation (5). In contrast, the coinhibitory checkpoint includes cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), and PD-1 ligand 1 (PD-L1) (6). CTLA-4 is highly homogenous to CD28 and has high binding capacity with B7-1 (CD80) and B7-2 (CD86). However, binding of CTLA-4 with B7 does not stimulate T cells (7). PD-1 and its ligand PD-L1 are also a key coinhibitory receptor which can lead to T cell inhibition through different signaling pathways (8,9). These immune checkpoints prevent the immune system from attacking healthy cells indiscriminately (10), however, sustained expression of CTLA-4/PD-1/PD-L1 could induce decreased T cell effector function (T cell exhaustion) and lead to cancer immune evasion (11).
Immune checkpoint inhibitors (ICIs) refer to monoclonal antibodies (mAbs) against CTLA-4/PD-1/PD-L1, which could activate T cell and kill cancer cells (6,12). These ICIs have been extensively used as anti-tumor agent in clinical practice (6,12). Among them, camrelizumab, a PD-1 antibody (13,14), has been used as first-line drug for the treatment of many cancers including lung cancer (15), hepatocellular carcinoma (16) gastric adenocarcinoma (17) and head/neck cancer (18). Camrelizumab-related side effects need much attention considering its wide usage in the treatment of cancers. For example, it has been reported that camrelizumab could induce immune-related adverse events (irAEs) including pneumonitis (19), hepatitis (20), myocarditis (21) and myositis (22). However, camrelizumab-related central neuropathy such as encephalitis is rare and no confirmed therapy has been reported.
It has been reported that lung cancer accounts for 85% of cancer cases, and diagnosis is mainly made in the late stage or metastasis of the disease (23). In the present study, we reported a patient, with squamous cell carcinoma (SCC) of the lung, a type of non-small cell lung cancer (NSCLC), showed typical encephalitis symptoms including systemic fatigue, numbness of extremities and walking instability one month after anti-tumor immunotherapy with camrelizumab. Importantly, the total protein in cerebrospinal fluid (CSF) was significantly elevated (1,399 vs. normal value 50–150 mg/L). It is concluded that encephalitis may be an irAE of camrelizumab in this patient. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-58/rc).
Case presentation
A timeline of the treatment is provided in Figure 1. At the beginning of November 2020, a 67-year-old man was found a mass in the upper lobe of the left lung during physical examination, and the postoperative pathological results showed that it was SCC in the upper lobe of the left lung. There were no financial, language or culture challenges during the diagnosis. The immunohistochemistry was as follows: NapsinA(−), p40(+), TTF-1(−), CgA(−), Syn(−), Ki-67(~50+), CK5/6(+), CK34βE12(+) (Figure 2). On 1 December 2020 and 25 January 2021, the patient received three cycles of carelizumab 200 mg + 400 mg paclitaxel (albumin binding type) + 500 mg carboplatin.


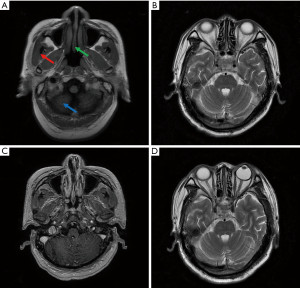
One month after the treatment (19 February 2021), the patient complained of general fatigue, numbness of extremities and unstable walking. No medical, family, and psycho-social history were noted for this patient. On 22 February 2021, the patient did not get better after two days of intravenous injection of neurotrophic drugs including thioctic acid (1.2 g) and cobamamide (1.5 mg) once daily. Then, further examinations were performed. Brain T2-fluid attenuated inversion recovery (T2-FLAIR) magnetic resonance imaging (MRI) showed that T2 weighted image (T2WI) and FLAIR sequences had a few patchy with high signal shadows in the parietal lobe of both frontal lobes, while these on T1 weighted image (T1WI) and FLAIR sequences were normal. No abnormal enhanced patchy was found with enhanced brain parenchyma. The shape, size and signal of the ventricular system were normal. The midline structure of the brain was in the middle. The shape, size, and signal of cistern and sulcus were normal. The structure of saddle area was normal. There were no pathological changes in the structure and signal of skull base. There was no brain metastasis of the tumor (Figure 3). On 3 March 2021, the patient showed an increased protein level of CSF (1,399 vs. normal value 50–150 mg/L) and glucose (4.7 vs. normal value 6.0 mmol/L). The patient reported no history of basic diseases such as diabetes, cardiovascular diseases, hyperlipidemia, cerebrovascular diseases or autoimmune diseases. Based on these results, this patient was diagnosed as immune-related encephalitis induced by carrelizumab.
Then, the patient was started with high-dose intravenous methylprednisolone (MP) on 3 March 2021. Days 1–4: 500 mg MP, the patient still felt numb in lower limbs, and his walking instability was slightly improved; days 5–10: 120 mg MP; days 11–15: 60 mg MP. After the treatments, the numbness of the patient’s limbs was better than before, and he could walk on the ground. From the 16th day, MP was stopped and prednisone acetate tablets 30 mg was given orally once daily to continue the treatment. Direct questioning of the patient was used to assess the intervention adherence and tolerability. On 23 March 2021, it was found that lactate dehydrogenase (LDH) and glucose in CSF increased slightly, and red cell count in CSF significantly decreased (Table 1). Of note, total protein in CSF decreased from 1,399 mg/L before treatment to 873 mg/L after treatment, indicating that the CSF protein level was significantly improved. In addition, white cell count and Cl− content in CSF remained unchanged compared with that measured on 3 March 2021. The follow-up one month later showed that immune-related encephalitis completely disappeared. During the last follow-up one year after the onset of immune-related encephalitis, the patient showed no recurrence of neurological symptoms. There were no adverse and unanticipated events after the usage of this treatment. Therefore, the present study may provide some useful information on the treatment and outcome of PD-1-induced encephalitis.
Table 1
| Parameters | 3 March 2021 | 23 March 2021 |
|---|---|---|
| Red cell count (/μL) | 186 | 26 |
| White cell count (/μL) | 10 | 10 |
| LDH (U/L) | 154 | 199 |
| Cl− (mmol/L) | 127 | 121 |
| Glucose (mmol/L) | 4.7 | 6.3 |
| Total protein (mg/L) | 1,399 | 873 |
LDH, lactate dehydrogenase.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Patient perspective
“One month after anti-tumor camrelizumab treatment, I felt fatigue, numbness in the limbs, and I was not able to walk stably. Then I went to The First People’s Hospital of Jiashan. After a series of examinations, I was diagnosed as camrelizumab-related encephalitis. I did not get better after intravenous injection of neurotrophic drugs. I was happy that the symptoms mentioned above completely disappeared after treatment with different dosage of MP.”
Discussion
In the present study, we reported the diagnosis and treatment of a patient with SCC of the lung. One month after the treatment with camrelizumab, this patient was clinically diagnosed as immune-related encephalitis as evidenced by increased CSF total protein, general asthenia, numbness of extremities and walking instability. This toxicity still occurred, although the dose of camrelizumab was only used for 3 cycles (200 mg). Of note, the patient’s clinical symptoms were totally relieved after treatment with high-dose MP and prednisone acetate.
The mechanisms and management of side effects of ICIs such as camrelizumab should be paid much attention, considering its wide application in the treatment of various cancers (24). It has been reported that there was sustained PD-1 expression in chronic inflammation, and the increased expression of PD-1 was transient in acute inflammation (25). Of note, sustained upregulation of PD-1 reduces T cell effector function and leads to T cell exhaustion (11). PD-1 has two structural motifs including one immunoreceptor tyrosine-based inhibitory motif (ITIM) and one immunoreceptor tyrosine-based switch motif (ITSM) (26). Upon binding with its ligands PD-L1 or PD-L2, ITIM and ITSM are phosphorylated at Y223 and Y248, respectively (26). PD-1 phosphorylation leads to the docking of many protein tyrosine phosphatases, including Src homology 2-containing protein tyrosine phosphatase 2 (SHP2), SHP2 then subsequently inhibited the phosphoinositide 3-kinase (PI3K)/Akt and Ras/MEK/ERK pathway (26). It has been demonstrated that Akt deactivation reduces T cell proliferation and increases apoptosis, thereby promoting T cell exhaustion, and decreased ERK1 activation reduces proliferation and differentiation potential (3). Then, PD-1-mediated T cell exhaustion fail to control tumor growth and promotes tumor survival and immune evasion (27,28). Therefore, PD-1 has been a promising target for the immune therapy of cancers (29,30).
However, much attention should be paid to irAEs of ICIs (31,32). The early identification and management of adverse reactions in the nervous system is crucial to maximize clinical recovery and minimize the impact of drug-related toxicity (31,32). There are many different ICIs-related irAEs covering nearly all organ systems including stomach, gut, lung, liver, heart and muscle (33,34). It has been demonstrated that the incidence of neurological irAEs is lower in ICIs monotherapy compared with combination therapy with two or more ICIs (35,36). In general, signs and symptoms often occur within 1 month of starting ICIs treatment (36), but the diagnosis of irAEs versus metastasis may be challenging in clinical practice (37). In the present study, we reported a case of immune-related encephalitis which occurred 1 month after camrelizumab treatment. The irAEs of encephalitis were diagnosed according to total CSF protein, general asthenia, numbness of extremities and walking instability. Importantly, we found no brain metastasis in MRI examination. The symptoms of irAEs were not relieved with neurotrophic drugs thioctic acid and cobamamide, whereas the symptoms were totally lost after treatment with MP and prednisone acetate. One year after the onset of encephalitis, the patient was in good condition without neurological symptom recurrence. Therefore, corticosteroids may be first drug for the treatment of irAEs. These neurological irAEs associated with immuno-oncotherapy should be paid more attention after the treatment as indicated in a previous meta-analysis (38).
The occurrence of irAE may be related to the enhanced immune response, although the exact mechanism of irAE remains largely unknown (36). Several hypotheses have been proposed: (I) there is some cross reactivity of T cells between tumor cells and normal tissue (39); (II) ICIs can increase the levels of previously existing autoantibodies, such as anti-thyroid antibodies (40); (III) ICIs can increase the levels of inflammatory cytokines; (IV) In organs that directly express CTLA-4 or PD-L1 to protect normal tissues, such as in normal pituitary or myocardial cells, ICI can interfere with these self-protection systems (40,41). Therefore, activated T cells may attack healthy tissues, leading to irAE similar to that of autoimmune diseases (42).
Conclusions
In summary, immune-related encephalitis is an irAE of camrelizumab. The present case proves the efficacy and safety of using corticoids in the treatment of encephalitis. However, larger-scale and longer-term studies are needed to investigate and confirm the natural course and treatment strategy of ICIs-induced encephalitis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-58/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-58/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-58/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leitinger M, Varosanec MV, Pikija S, et al. Fatal Necrotizing Encephalopathy after Treatment with Nivolumab for Squamous Non-Small Cell Lung Cancer: Case Report and Review of the Literature. Front Immunol 2018;9:108. [Crossref] [PubMed]
- Shah K, Al-Haidari A, Sun J, et al. T cell receptor (TCR) signaling in health and disease. Signal Transduct Target Ther 2021;6:412. [Crossref] [PubMed]
- Jubel JM, Barbati ZR, Burger C, et al. The Role of PD-1 in Acute and Chronic Infection. Front Immunol 2020;11:487. [Crossref] [PubMed]
- Mariuzza RA, Agnihotri P, Orban J. The structural basis of T-cell receptor (TCR) activation: An enduring enigma. J Biol Chem 2020;295:914-25. [Crossref] [PubMed]
- Esensten JH, Helou YA, Chopra G, et al. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016;44:973-88. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Xia F, Qian CR, Xun Z, et al. TCR and CD28 Concomitant Stimulation Elicits a Distinctive Calcium Response in Naive T Cells. Front Immunol 2018;9:2864. [Crossref] [PubMed]
- He X, Xu C. PD-1: A Driver or Passenger of T Cell Exhaustion? Mol Cell 2020;77:930-1. [Crossref] [PubMed]
- Chikuma S. Basics of PD-1 in self-tolerance, infection, and cancer immunity. Int J Clin Oncol 2016;21:448-55. [Crossref] [PubMed]
- Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492-9. [Crossref] [PubMed]
- Alturki NA. Review of the Immune Checkpoint Inhibitors in the Context of Cancer Treatment. J Clin Med 2023;12:4301. [Crossref] [PubMed]
- Markham A, Keam SJ. Correction to: Camrelizumab: First Global Approval. Drugs 2019;79:1497. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. [Crossref] [PubMed]
- Ren S, Chen J, Xu X, et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J Thorac Oncol 2022;17:544-57. [Crossref] [PubMed]
- Xia Y, Tang W, Qian X, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer 2022;10:e004656. [Crossref] [PubMed]
- Peng Z, Wei J, Wang F, et al. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2021;27:3069-78. [Crossref] [PubMed]
- Zhang Z, Wu B, Peng G, et al. Neoadjuvant Chemoimmunotherapy for the Treatment of Locally Advanced Head and Neck Squamous Cell Carcinoma: A Single-Arm Phase 2 Clinical Trial. Clin Cancer Res 2022;28:3268-76. [Crossref] [PubMed]
- Li L, Lou A, Yu J. Immune checkpoint inhibitor-related pneumonitis induced by camrelizumab: a case report and review of literature. Ann Palliat Med 2021;10:8460-6. [Crossref] [PubMed]
- Tan Y, Ye Y, Chen L. Fatal immune-related hepatitis with intrahepatic cholestasis and pneumonia associated with camrelizumab: A case report and literature review. Open Med (Wars) 2021;16:553-7. [Crossref] [PubMed]
- Liang S, Yang J, Lin Y, et al. Immune Myocarditis Overlapping With Myasthenia Gravis Due to Anti-PD-1 Treatment for a Chordoma Patient: A Case Report and Literature Review. Front Immunol 2021;12:682262. [Crossref] [PubMed]
- Bai J, Li D, Yang P, et al. Camrelizumab-Related Myocarditis and Myositis With Myasthenia Gravis: A Case Report and Literature Review. Front Oncol 2021;11:778185. [Crossref] [PubMed]
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- Song H, Liu X, Jiang L, et al. Current Status and Prospects of Camrelizumab, A Humanized Antibody Against Programmed Cell Death Receptor 1. Recent Pat Anticancer Drug Discov 2021;16:312-32. [Crossref] [PubMed]
- Shwetank, Frost EL, Mockus TE, et al. PD-1 Dynamically Regulates Inflammation and Development of Brain-Resident Memory CD8 T Cells During Persistent Viral Encephalitis. Front Immunol 2019;10:783.
- Patsoukis N, Duke-Cohan JS, Chaudhri A, et al. Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation. Commun Biol 2020;3:128. [Crossref] [PubMed]
- Mizuno R, Sugiura D, Shimizu K, et al. PD-1 Primarily Targets TCR Signal in the Inhibition of Functional T Cell Activation. Front Immunol 2019;10:630. [Crossref] [PubMed]
- Budimir N, Thomas GD, Dolina JS, et al. Reversing T-cell Exhaustion in Cancer: Lessons Learned from PD-1/PD-L1 Immune Checkpoint Blockade. Cancer Immunol Res 2022;10:146-53. [Crossref] [PubMed]
- Pezeshki PS, Rezaei N. Immune checkpoint inhibition in COVID-19: risks and benefits. Expert Opin Biol Ther 2021;21:1173-9. [Crossref] [PubMed]
- Yang T, Li W, Huang T, et al. Immunotherapy Targeting PD-1/PD-L1 in Early-Stage Triple-Negative Breast Cancer. J Pers Med 2023;13:526. [Crossref] [PubMed]
- Matsuoka H, Hayashi T, Takigami K, et al. Correlation between immune-related adverse events and prognosis in patients with various cancers treated with anti PD-1 antibody. BMC Cancer 2020;20:656. [Crossref] [PubMed]
- Liu Y, Wang H, Deng J, et al. Toxicity of tumor immune checkpoint inhibitors-more attention should be paid. Transl Lung Cancer Res 2019;8:1125-33. [Crossref] [PubMed]
- Darnell EP, Mooradian MJ, Baruch EN, et al. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr Oncol Rep 2020;22:39. [Crossref] [PubMed]
- Pathak R, Katel A, Massarelli E, et al. Immune Checkpoint Inhibitor-Induced Myocarditis with Myositis/Myasthenia Gravis Overlap Syndrome: A Systematic Review of Cases. Oncologist 2021;26:1052-61. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Choi J, Lee SY. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw 2020;20:e9. [Crossref] [PubMed]
- Kostine M, Finckh A, Bingham CO, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis 2021;80:36-48. [Crossref] [PubMed]
- Chen W, Chen J, Zhang L, Cheng S, Yu J. Network meta-analysis of first-line immune checkpoint inhibitor therapy in advanced non-squamous non-small cell lung cancer patients with PD-L1 expression ≥ 50. BMC Cancer 2023;23:791. [Crossref] [PubMed]
- Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 2017;123:2143-53. [Crossref] [PubMed]
- Kimbara S, Fujiwara Y, Iwama S, et al. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci 2018;109:3583-90. [Crossref] [PubMed]
- Baban B, Liu JY, Qin X, et al. Upregulation of Programmed Death-1 and Its Ligand in Cardiac Injury Models: Interaction with GADD153. PLoS One 2015;10:e0124059. [Crossref] [PubMed]
- Ibis B, Aliazis K, Cao C, et al. Immune-related adverse effects of checkpoint immunotherapy and implications for the treatment of patients with cancer and autoimmune diseases. Front Immunol 2023;14:1197364. [Crossref] [PubMed]
Cite this article as: Wang YY, Song JJ. A case report of the diagnosis and treatment of immune checkpoint inhibitor-related encephalitis induced by camrelizumab. AME Case Rep 2024;8:101.


