
Traumatic chylothorax: a case report, treatment options and an update of the literature
Highlight box
Key findings
• The study presents a case of traumatic chylothorax following a fracture of the Th12 vertebra, detailing successful diagnosis and comprehensive treatment approaches, including nutritional support and surgical intervention, leading to the patient’s recovery.
What is known and what is new?
• Chylothorax can result from neoplastic processes or surgical injuries, with traditional management involving dietary modifications, thoracic duct ligation, or pleurodesis.
• This manuscript highlights a rare case of chylothorax due to spinal trauma, emphasizing the role of early diagnosis, the effectiveness of a medium-chain fatty acid diet, and innovative surgical techniques in managing traumatic chylothorax.
What is the implication, and what should change now?
• The findings underscore the necessity for heightened awareness and prompt management of chylothorax post-spinal trauma. It advocates for incorporating advanced diagnostic and therapeutic strategies with a multidisciplinary approach, including the use of video-assisted thoracic surgery for thoracic duct identification and ligation, and the potential of dietary management to mitigate chylous leakage. This case encourages a reevaluation of current treatment protocols to integrate these insights for improving patient outcomes.
Introduction
Chylothorax, an uncommon condition, occurs when lymph fluid leaks into the pleural space due to damage to lymphatic channels or the thoracic duct. This condition can lead to several complications, such as difficulties in breathing, nutritional deficiencies, and weakened immunity due to lymphopenia (1,2). Frequent etiologies of chylothorax include malignant and non-malignant causes, such as cirrhosis, nephrosis, congestive heart failure, and unintentional damage to the thoracic duct in the course of surgical procedures (3,4). Herein, we elaborate on a case of chylothorax precipitated by a fracture at the Th12 vertebra, examining the diagnostic measures and treatment methodologies. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-34/rc).
Case presentation
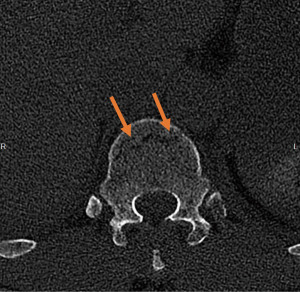
A male patient in his 60s with a history of hypercholesterolemia was brought to the emergency room after a cycling accident hitting his spine against a lamppost. At the emergency department the initial chest X-ray suggested a minimal amount of fluid and a fracture of costa 11 on the right side (Figure 1). The additional total body computed tomography (CT) showed a fracture of the anterior column of the Th12 vertebra [Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification—type B2: a vertebral body facture with disruption of the posterior ligamentous complex] and fractures of the transvers process of Th10, L1 to L4 and the spinous process of Th11 and L2 on the left side (Figure 2). Moreover, a posterior fracture of costa 10 to 12 on the left and a fracture of costa 11 on the right side were seen. At last, the CT angiography of the chest showed a hematoma of the mediastinum at the level of the Th10–11 vertebra without active bleeding, and a contusion of the lung with some pleural effusion on the right. There was no pneumothorax. The patient was admitted to the intensive care unit for observation and the next day the patient underwent a minimally invasive percutaneous dorsal pedicle screw-rod spondylodesis of Th11 and L1 for stabilization of the Th12 vertebra. No intra-operative complications were observed.

The day following surgery, the patient exhibited escalating shortness of breath and diminished breath sounds in the right lung area upon examination. Subsequent chest radiography revealed an increased right-sided pleural effusion (Figure 3). Vital signs and hemoglobin levels remained stable (8.8 mmol/L). A CT angiogram was conducted, revealing significant fluid accumulation within the right pleural space (Figure 4). There were no signs of postoperative bleeding. A chest tube was placed and a total of 3 L of serosanguineous pleural fluid with a lipid content was evacuated. The patient’s condition markedly enhanced following the drainage of the effusion, and the extracted fluid was dispatched for biochemical analysis.

Analysis of the pleural fluid indicated a pH of 7.45, total protein concentration of 32 g/L, lactate dehydrogenase (LDH) at 217 IU/L, and triglycerides measuring 28.6 mmol/L (2,531.0 mg/dL). No bacterial growth was observed, and cytologic analysis was not performed. The diagnosis of chylothorax was established based on the fluid’s biochemistry, with cholesterol levels being less than 5.18 mmol/L and triglyceride levels exceeding 1.24 mmol/L, which are indicative of chylous pleural effusion (2,5). According to the Light criteria [pleural/serum LDH: 217/169 =1.3 (≥0.6) and pleural LDH (217) >2/3 × upper limit of normal serum LDH (250): >2/3×250 =167 (167/217 =0.8 (≥0.6)] and the Heffner criteria [pleural protein = 3.2g/dL (>2.9 g/dL)] the pleural effusion was classified as exudative effusion (6,7).
In the following days, a chylous production up to 3 L/24 h was observed. On the 5th day after the chest tube was placed, the patient started on a diet consisting of medium-chain triglyceride (MCT), and 8 days later, total parenteral nutrition (TPN) was initiated. The production of chylous remained consistently between 2 to 3 L per 24 hours in the first 2 weeks. On the 16th day, due to the ongoing chylous leakage, despite the MCT and TPN diet without enteral feeding, the patient underwent a video-assisted thoracic surgery (VATS); the thoracic duct was identified and clipped. In addition, a chemical pleurodesis with talc was performed. The procedure was complicated with a small chylous empyema (confirmed by CT) which was successfully drained and treated with antibiotics. After a total of 7 weeks (4 weeks after surgery) the patient was discharged from our hospital. The MCT diet was continued for another 2 weeks at home (a total of 8 weeks MCT—6 weeks after surgery) after which he started enteral feeding. At the outpatient clinic, 1 month after discharge (3 months after the initial trauma) from our hospital the patient was further clinically improving, both physically and mentally. A follow-up chest X-ray showed only minimal pleural effusion. On physical examination at the outpatient clinic there was a small reduced ability to expand his right hemithorax compared to the left without any signs of respiratory distress—probably due to the trauma and postoperative scar tissue.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Discussion
The thoracic duct, originating from the cisterna chyli located just before the first or second lumbar vertebra, ascends through the aortic hiatus into the posterior mediastinum. It lies right of the midline between the aorta and the azygos vein between the twelfth and eighth thoracic vertebrae. The duct crosses to the left side between the sixth and fourth thoracic vertebrae, moving behind the esophagus into the left posterior mediastinum. It arches into the superior mediastinum and descends to empty near the junction of the left internal jugular and subclavian veins. The thoracic duct’s anatomical path is crucial for understanding chylothorax occurrences: injury below the Th5–6 level often causes a right-sided chylothorax due to the duct’s position on the right at those levels. In contrast, damage above this level can result in a left-sided chylothorax, as the duct crosses over to the left higher in the thorax. This distinction is essential for diagnosing and treating chylothorax, highlighting the importance of the thoracic duct’s location and trajectory within the body (8).
Non-iatrogenic causes of chylothorax such as blunt trauma are responsible for only 20% of cases of traumatic chylothorax. Most cases are iatrogenic and result from damage to the thoracic duct during elective surgery including esophagectomy and lung surgery (2,9). If non-iatrogenic chylothorax does occur after blunt trauma, it frequently correlates with fractures of the posterior ribs and/or vertebrae, as observed in this instance (2,10). While chylothorax caused by blunt trauma is uncommon, it can lead to severe complications with mortality rates as high as 50% if not managed properly. The diagnosis relies on the lipid concentration in the pleural fluid, as previously mentioned, and the detection of chylomicrons in the fluid serves as an additional diagnostic marker when testing capabilities are present (2,5). In most cases chylothorax can be classified as exudative. Transudative chylothorax is extremely rare, but does occur and can be secondary to underlying disease, such as cirrhosis, nephrosis and congestive heart failure. The Light criteria and the Heffner criteria can be used to classify pleural effusion to either transudative or exudative effusion. In this case report both criteria and were used as Light’s criteria alone could misinterpret up to 25% of transudative effusion as exudative. According to both the Light and the Heffner criteria the pleural effusion in our patient was classified as exudative. Two out of 3 criteria of Light’s and Heffner’s criteria could be calculated as total serum protein and pleural cholesterol levels were not recorded (6,7).
It is vital to provide robust nutritional support for patients who have suffered extensive loss of lipid and lymphocyte-containing fluid as a result of chyle leakage, in order to prevent malnutrition, dehydration, and immune function complications (8). A diet predominantly composed of MCT is effective in reducing lymphatic flow, which in turn can lower the incidence of chyle leakage. MCT are taken up directly into the portal circulation from the intestinal system, unlike long-chain triglycerides, which are processed into chylomicrons and transported through the intestinal lymph vessels (11). These lymph vessels lead to the thoracic duct and eventually merge with the bloodstream at the subclavian vein. When a chylothorax is sufficiently large to impede breathing, the insertion of an intercostal chest drain becomes necessary (9). This intervention also allows for precise tracking of chyle drainage volumes. It has been suggested that in the initial phase of a chylothorax, a non-surgical approach involving complete fasting coupled with TPN for a duration of two weeks can successfully manage low-output lymphatic leaks (12). In cases were the chyle output remains high, despite administration of a MCT diet, octreotide (a somatostatin analogue) has been shown to be successful by reducing intestinal chyle production and therefore reducing leakage by the damaged duct (5,13,14). Although no randomized controlled trials have been performed assessing its effectiveness, octreotide could be useful as an adjunct to conservative treatment.
If conservative treatment remains ineffective, thoracic duct ligation can be performed with either a thoracotomy or VATS. The patient in our case report underwent a VATS. Preoperatively, no lymphangiography or enteral administration of fat with methylene blue was performed to localize the point of leakage. During VATS the point of injury was suggested to be between the tenth and twelfth vertebrae on the right side in close proximity of the fractured Th12 vertebra. Posterodorsal the parietal pleura was opened to enter the posterior mediastinum. Dorsal to the esophagus the thoracic duct was identified. Because no clear point of injury could be identified and to avoid further damage to the duct, we decided to perform a mass ligation by clipping the thoracic duct. In addition, a talc pleurodesis was conducted to improve the overall chance of success. Reports indicate that duct ligation above the level of the right hemidiaphragm has success rates as high as 90% (5,9). Moreover, the use of fluorescence-guided techniques during VATS with a near-infrared (NIR) light imaging system enhances visualization of the thoracic duct. This is achieved by administering indocyanine green (ICG), which helps to trace the pathway of the thoracic duct and pinpoint the exact site of leakage (12,15). If locating the thoracic duct proves unsuccessful or if attempts to clip the duct are not effective, a chemical pleurodesis using talc may be conducted, which appears to have a high success rate according to multiple case series (9). Embolization of the thoracic duct is an important alternative in treatment of patients with persistent traumatic and non-traumatic chylous leaks with success rates up to 84% with coiling alone, and up to 91% when liquid embolic agents were added (15,16).
Conclusions
In conclusion, while it is uncommon, spinal trauma may lead to the disruption of the thoracic duct, resulting in chylothorax. The diagnosis of chylothorax primarily relies on detecting elevated triglyceride levels in the chylous fluid and the presence of chylomicrons when triglyceride concentrations are inconclusive. Therapeutic approaches for ongoing chylothorax include an array of methodologies, spanning conservative medical management to more aggressive radiological and surgical techniques.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-34/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-34/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hillerdal G. Chylothorax and pseudochylothorax. Eur Respir J 1997;10:1157-62. [Crossref] [PubMed]
- Sendama W, Shipley M. Traumatic chylothorax: A case report and review. Respir Med Case Rep 2015;14:47-8. [Crossref] [PubMed]
- Akbar A, Hendrickson T, Vangara A, et al. Hepatic Chylothorax: An Uncommon Pleural Effusion. J Investig Med High Impact Case Rep 2023;11:23247096221150634. [Crossref] [PubMed]
- Do TVC, Cozza J, Ganti S, et al. Recurrent Chylous Ascites Leading to Transudative Chylothorax Due to Bi-Ventricular Heart Failure. J Investig Med High Impact Case Rep 2021;9:23247096211026144. [Crossref] [PubMed]
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med 2010;104:1-8. [Crossref] [PubMed]
- Vangara A, Haroon M, Kalafatis K, et al. Chylothorax in the Setting of Lung Malignancy. J Investig Med High Impact Case Rep 2023;11:23247096231192876. [Crossref] [PubMed]
- Devkota KC, Hamal S, Panta PP. Comparison of Heffner criteria and Light criteria in differentiating exudative and transudative pleural effusion. Nepal Med Coll J 2020;22:141-5. [Crossref]
- Valentine VG, Raffin TA. The management of chylothorax. Chest 1992;102:586-91. [Crossref] [PubMed]
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg 2007;32:362-9. [Crossref] [PubMed]
- Townshend AP, Speake W, Brooks A. Chylothorax. Emerg Med J 2007;24:e11. [Crossref] [PubMed]
- Merrigan BA, Winter DC, O'Sullivan GC. Chylothorax. Br J Surg 1997;84:15-20. [PubMed]
- Londero F, Grossi W, Vecchiato M, et al. Fluorescence-Guided Identification of the Thoracic Duct by VATS for Treatment of Postoperative Chylothorax: A Short Case Series. Front Surg 2022;9:912351. [Crossref] [PubMed]
- Collard JM, Laterre PF, Boemer F, et al. Conservative treatment of postsurgical lymphatic leaks with somatostatin-14. Chest 2000;117:902-5. [Crossref] [PubMed]
- Sharkey AJ, Rao JN. The successful use of octreotide in the treatment of traumatic chylothorax. Tex Heart Inst J 2012;39:428-30. [PubMed]
- Bazancir LA, Jensen RJ, Frevert SC, et al. Embolization of the thoracic duct in patients with iatrogenic chylothorax. Dis Esophagus 2021;34:doab001. [Crossref] [PubMed]
- Chen E, Itkin M. Thoracic duct embolization for chylous leaks. Semin Intervent Radiol 2011;28:63-74. [Crossref] [PubMed]
Cite this article as: de Goede B, de Jong L, van Rossem CC, Schep NWL. Traumatic chylothorax: a case report, treatment options and an update of the literature. AME Case Rep 2024;8:105.



