
Isolated central nervous system (CNS) relapse of multiple myeloma 11 years after autologous stem cell transplantation: a case report
Highlight box
Key findings
• Intrathecal (IT) chemotherapy with methotrexate and dexamethasone plus a systemic pomalidomide-bases regimen can be safe and effective in the very rare scenario of an isolated central nervous system (CNS) relapse of multiple myeloma.
What is known and what is new?
• Isolated CNS relapse after autologous stem cell transplant is rare, a matter of only case reports. Therefore, a standard treatment has not been established. However, based on preclinical and a few clinical studies we know that immunomodulatory drugs can be effective.
• With this manuscript, we increase the clinical experience that demonstrates the effectiveness of a combination of IT chemotherapy plus a pomalidomide-based regimen.
What is the implication, and what should change now?
• Feasible and available treatments in contexts where the newer therapies (bispecific, chimeric antigen receptor-T cells) might not be widely available should be explored in this unusual context.
Introduction
Multiple myeloma (MM) relapse in the central nervous system (CNS) is rare, less than 1%, when it occurs a systemic relapse is almost always documented, and usually happens in a very short period after initial treatment and response, between 6 and up to 36 months in most cases. Patients usually have a very short overall survival (OS), less than 6 months (1,2).
Even though each year there are drugs approved for treatment of the disease, treatment for CNS involvement, due to its rarity, is not yet defined.
Herein we report the case of a patient with a CNS MM relapse after stringent complete response (sCR), 11 years after ASCT. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-19/rc).
Case presentation
A 49-year-old-made, presented in October 2009 with an IgG lambda MM, ISS II. He had a history of hypertension and poorly controlled type 2 diabetes mellitus; he had undergone a 3-year course of adalimumab for ankylosing spondylitis. He was treated with bortezomib/dexamethasone and autologous stem cell transplant (ASCT), and then a 2 years maintenance period with thalidomide, he attained sCR, which was confirmed by a bone marrow biopsy and positron emission tomography/computed tomography (PET/CT).
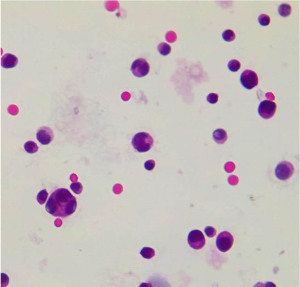
He disappeared to follow-up and returned in June 2021, at the age of 59 years, after 11 years and 8 months of progression-free survival (PFS), with bilateral lower limb paraplegia, clinical findings were consistent with Cauda Equina syndrome. Nuclear magnetic resonance (NMR) showed leptomeningeal infiltration (Figure 1), a single focal intramedullary lesion at L1 level without bone affectation was also found. He had none of the MM defining criteria (CRAB). His serum immunoglobulins (IGs) were in the normal range, he also had both serum and urine immunofixation negative and normal levels of serum-free light chains. Flow cytometry in bone marrow did not show an aberrant population of plasma cells. Lumbar puncture was unsuccessful and through an Ommaya reservoir, his cerebrospinal fluid (CSF) analysis showed infiltration by countless malignant plasma cells (Figure 2).

He received urgent conformational radiotherapy with a 20 Gy dose, followed by twice a week intrathecal (IT) chemotherapy with methotrexate and hydrocortisone for seven doses which attained progressive disappearance of aberrant plasma cells in CSF, after that we continued to complete 19 weekly doses with the same agents. One month after we started treatment, his course was complicated with a leg proximal deep vein thrombosis concomitant with a pulmonary embolism that required anticoagulation with apixaban. He then received 12 cycles of pomalidomide/dexamethasone with progressive clinical improvement. The CSF was repetitively acellular. He recovered 80% motor function in his lower extremities, he was able to stand and walk with some assistance. Unfortunately, in June 2022, anticoagulation was halted because he underwent an upper gastrointestinal (GI) endoscopic procedure, and upon returning home he had a sudden death, likely due to pulmonary embolism. A full timeline of this case is contained in Figure 3.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Discussion
Extramedullary disease in MM is more frequent at relapse than at initial diagnosis (10–30% vs. 6–8%) (1), and it is almost always associated with systemic relapse.
CNS relapse after ASCT is even rarer, there is only a few cases reported, none of them beyond 10 years.
There are a few risk factors that have been identified among these relapsed patients: lambda subtype, elevated lactate dehydrogenase (LDH) and β-2 microglobulin, plasma cell leukemia, other extramedullary sites and cytogenetic abnormalities such as 17p deletion and 13q deletion (2). In this case, we unfortunately could not obtain cytogenetic analysis since the cells were exclusively found in the CSF, therefore the risk was not stratified.
However, we showed both clinical and laboratory improvement with a feasible treatment in our context, where monoclonal antibodies and cellular therapy are not widely available.
As of today, there have been 14 cases of isolated CNS relapse of MM in patients that were treated with ASCT (1-8) have been reported. With a median time of relapse between ASCT and CNS disease of 6 months (2.5–84 months), and a median OS of also 6 months (0.3–29 months).
Two of these patients had a survival of less than a month and a survival longer than 1 year after the CNS relapse was reported in only one patient (2,3). Most of them had an ISS stage III, and abnormal cytogenetics.
One case was controlled and the patient received a second autologous transplant but presented a leptomeningeal relapse 9 months post-transplant (5). Two of the 14 cases had an initial diagnosis of plasma cell leukemia (4,5). Eventually, 3 of the 14 relapsed cases developed systemic affectation, the rest remained isolated to the CNS. Seven had tumoral masses in addition to meningeal infiltration.
Treatment requires drugs that cross blood-brain barrier (BBB). Some chemotherapy agents can do so, methotrexate, cytarabine, thiotepa among others, however they are not effective as myeloma treatment. Despite this, IT chemotherapy was given in the aforementioned cases. We know that alkylating agents such as cyclophosphamide and melphalan do not significantly cross the BBB.
Plasma cells are radiosensitive, so parenchymal tumors can be treated with this strategy, with hematology toxicity being a concern.
Thalidomide, the first member of the immunomodulatory drugs (IMiDs) has been detected in CSF after oral administration (6), it is amongst the few medications, with corticosteroids, with this characteristic, however, in this case the potential neuropathy with a spinal tumor left it out as an option for us. Lenalidomide and pomalidomide have a penetration to CSF of 11% and 49%, respectively. The second one has proven its efficacy in clinical scenarios (8,9) and even been used as a bridging therapy before other potential curative strategies (10).
With respect to proteasome inhibitors, marizomib and carfilzomib can cross the BBB, both have proven adequate clearance of plasma cells in the CSF (11). Bortezomib has no significant effect (12). Marizomib showed clinical improvement in 2 patients treated with a MM CNS relapse, one isolated and one in the context of a systemic relapse (13).
As for anti CD38 monoclonal antibodies, there have been however some reports of SNC disease with durable and sustained reports of patients treated with daratumumab (14), nonetheless a post autologous transplant case relapsed during daratumumab maintenance and mass spectrometry obtained at 12, 15 and 19 days after the monoclonal antibody infusion demonstrated CSF levels that were up to 71 times smaller than in the serum those same days.
It is well known that Selinexor can penetrate the BBB in up to 72% in murine models, reports also exist of successful treatment of MM with combinations including this agent (15), toxicities are a concern, and it is not available in our country.
Anti-BCMA receptor chimeric antigen receptor-T (CAR-T) cells have been developed for the treatment of MM, in a few cases with CNS infiltration responses have been obtained with either clinical response or as defined by the International Myeloma Working Group (IMWG) criteria (16-18).
Finally, as for bispecifics only with elranatamab, there is a report of one case of successful treatment (19).
In our country, the access to daratumumab and bispecifics is still limited, while CAR-T cells are not yet available.
Even though cytogenetics couldn’t be obtained at the first diagnosis, due to lack of resources, or this isolated relapse, due to lack of disease in bone marrow, we could hypothesize that the cause of this late presentation was a different, more aggressive clone that responded well to a therapy that crosses the BBB.
Implications and actions needed.
The effectiveness of combinations of steroids with IMiDs should be evaluated in prospective studies with a large number of subjects in order to conclude that this should be the standard of management of isolated CNS involvement by MM.
Conclusions
Herein we present an extremely rare case of an isolated relapse of MM to the SNC, due to the PFS after an ASCT, to our knowledge, the longest reported.
The best treatment strategy is yet to be established. Here we demonstrated that pomalidomide-based treatment is safe and effective, as our patient showed clinical improvement, further clinical data is necessary to confirm this finding. Also, yet to be established, as they have not been proved in this specific scenario, is the role of bispecific antibodies and CAR-T cells, but in regions where they might not be available, other strategies, like ours, must be reported and evaluated.
Acknowledgments
The authors would like to thank Alejandra Zárate-Osorno and Adrían Alejandro Carballo-Zárate for their contributions in the pathology field.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-19/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-24-19/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-19/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood 2016;127:971-6. [Crossref] [PubMed]
- Seftel MD, Maguire J, Voss N, et al. Intra-cerebral relapse following prolonged remission after autologous stem cell transplantation for multiple myeloma. Leuk Lymphoma 2002;43:2399-403. [Crossref] [PubMed]
- Laribi K, Mellerio C, Baugier A, et al. Meningeal involvement in multiple myeloma. Clin Case Rep 2015;3:84-7. [Crossref] [PubMed]
- Nakamura F, Narimatsu H, Furukawa K, et al. Central nervous system relapse after autologous peripheral blood stem cell transplantation in primary plasma cell leukemia. Jpn Med Assoc J 2006;49:324-6.
- De Jesus O. Multiple myeloma extramedullary relapse at the sellar and suprasellar region after autologous stem cell transplantation. Surg Neurol Int 2024;15:13. [Crossref] [PubMed]
- Li X, Wang W, Zhang X, et al. Multiple myeloma with isolated central nervous system relapse after autologous stem cell transplantation: A case report and review of the literature. Front Oncol 2022;12:1027585. [Crossref] [PubMed]
- Mittal A, Pushpam D, Kumar L. Isolated central nervous system relapse of multiple myeloma post autologous stem cell transplant- A rare presentation. Leuk Res Rep 2020;14:100207. [Crossref] [PubMed]
- Egan PA, Elder PT, Deighan WI, et al. Multiple myeloma with central nervous system relapse. Haematologica 2020;105:1780-90. [Crossref] [PubMed]
- Mussetti A, Dalto S, Montefusco V. Effective treatment of pomalidomide in central nervous system myelomatosis. Leuk Lymphoma 2013;54:864-6. [Crossref] [PubMed]
- Zhang Q, Zu C, Ni F, et al. Pomalidomide-based regimens bridging CAR-T therapy in multiple myeloma with central nervous system involvement. Regen Ther 2022;21:34-6. [Crossref] [PubMed]
- Williamson MJ, Blank JL, Bruzzese FJ, et al. Comparison of biochemical and biological effects of ML858 (salinosporamide A) and bortezomib. Mol Cancer Ther 2006;5:3052-61. [Crossref] [PubMed]
- Mele G, Pinna S, Alloro E, et al. Inefficacy of bortezomib therapy for CNS involvement of refractory multiple myeloma. Leuk Res 2007;31:721-3. [Crossref] [PubMed]
- Badros A, Singh Z, Dhakal B, et al. Marizomib for central nervous system-multiple myeloma. Br J Haematol 2017;177:221-5. [Crossref] [PubMed]
- Elhassadi E, Murphy M, Hacking D, et al. Durable treatment response of relapsing CNS plasmacytoma using intrathecal chemotherapy, radiotherapy, and Daratumumab. Clin Case Rep 2018;6:723-8. [Crossref] [PubMed]
- Fernandez LG 3rd, Oyon DE, Gondi V, et al. Secondary CNS myeloma with remission after systemic CNS-penetrating agents. Neurooncol Adv 2022;4:vdac106. [Crossref] [PubMed]
- Wang T, He T, Ma L, et al. Clinical Outcomes of BCMA CAR-T Cells in a Multiple Myeloma Patient With Central Nervous System Invasion. Front Oncol 2022;12:854448. [Crossref] [PubMed]
- Wang Y, Zu C, Teng X, et al. BCMA CAR-T Therapy Is Safe and Effective for Refractory/Relapsed Multiple Myeloma With Central Nervous System Involvement. J Immunother 2022;45:25-34. [Crossref] [PubMed]
- Wang Y, Wang L, Zeng Y, et al. Successful BCMA CAR-T Therapy for Multiple Myeloma With Central Nervous System Involvement Manifesting as Cauda Equina Syndrome-A Wandering Road to Remission. Front Oncol 2021;11:755584. [Crossref] [PubMed]
- Mutlu YG, Yıgıt Kaya S, Maral S, et al. Relapsed refractory multiple myeloma with CNS involvement successfully treated with Elranatamab: first reported case. Front Immunol 2023;14:1276295. [Crossref] [PubMed]
Cite this article as: Reynoso-Gómez EE, Quintero-Hernández CE. Isolated central nervous system (CNS) relapse of multiple myeloma 11 years after autologous stem cell transplantation: a case report. AME Case Rep 2024;8:111.


