
Trans-septal left ventricular endocardial lead in a patient with extensive anterior myocardial infarction and left ventricle (LV) apical endoventriculoplasty using a Vascutek patch—case report
Highlight box
Key findings
• In selected patients, where a tradition left ventricle (LV) lead epicardially through the coronary sinus (CS) fails. Tran-septal left ventricle (TSLV) lead implant is a safe alternative technique.
What is known and what is new?
• An optimal placement of the LV lead is crucial for the intended hemodynamic and clinical benefits. A small proportion of patients fail appropriate cardiac resynchronization therapy (CRT) either due to challenging CS access or suitable tributary.
• A well-localized target area and tools that help to achieve successful lead implantation seem to be of utmost importance to reach an optimal CRT. TSLV lead implant remains a viable alternative technique in those with failed trans-venous conventional CRT.
What is the implication, and what should change now?
• Response to CRT is essential. It improves symptoms of heart failure, reduces mortality and improves quality of life. Although a complex procedure, TSLV lead implant should be considered in selected patients.
Introduction
Alternative approaches have been used in patients, following failed conventional trans-venous left ventricle (LV) lead implant, these include trans-septal left ventricle (TSLV) lead, surgical approach to implant an epicardial lead, leadless LV endocardial lead and in recent times, conduction system pacing has been considered as a good alternative (1-11).
In a large study by Gamble et al., involving 29,503 patients, the overall rate of failed LV lead placement was 3.6%, this included inability to cannulate the coronary sinus (CS), unsuitable target vein and phrenic nerve stimulation (12). The viability and significance of scar tissue also lead to non-response to cardiac resynchronization therapy (CRT) or high LV lead threshold, making LV leads inefficient even if they have been well placed anatomically into a suitable CS branch (13).
Since CRT improves symptoms of heart failure, and reduces mortality and rate of hospitalisation (14,15). Alternative approaches are considered in patients who fail a traditional LV lead implant.
We present, a case report of a patient for where we successfully used a TSLV endocardial lead implantation approach following a failed LV lead implant via the CS. We present this case in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-23-109/rc).
Case presentation
This is a case report of a 78-year-old gentleman, ex-smoker, with complex past medical history. In 1975, aged 30 years old, he had an anterior myocardial infarction. He later developed left ventricular systolic dysfunction (LVSD) and apical aneurysm of the LV as a result of the ostial occlusion of the left anterior descending artery (LAD). This was further complicated by recurrent incessant ventricular tachycardia for which eventually, a surgical aneurysmectomy, endoventriculoplasty with Vascutek patch and surgical ablation of the ventricular tachycardia were performed successfully at Newcastle University Hospital in 1996. This was very successful in preventing recurrent ventricular tachycardia. Following this surgery, on a routine computed tomography (CT) scan, a suspected right lower lobe lesion was noted which was later confirmed to be a Blesowky tumour with benign features.
The patient developed chest pain, in 2011; he had a CT cardiac angiogram which revealed an already proximally occluded LAD and moderate disease to the left circumflex artery. A dobutamine stress echocardiogram revealed no inducible ischaemia within the circumflex artery territory and aneurysmal LAD territory, involving almost 50% of the LV. Patients’ symptoms were managed medically. At this time, he had no features of heart failure. His angina persisted, so he was brought in for a diagnostic invasive coronary angiogram and pressure wire study of the circumflex artery. This was positive hence a 2.25 mm × 15 mm stent was deployed with an excellent final outcome.
In 2019, he was admitted with features of decompensated heart failure, an echocardiogram revealed severely impaired LV systolic dysfunction with an ejection fraction of 15%. Since his electrocardiogram (ECG) showed left bundle branch block morphology with QRS duration of 175 months, he was listed for a CRT implantable cardioverter defibrillator (ICD), to safeguard him from ventricular arrhythmia and improve LV function.
The CRT ICD implant procedure was undertaken in March 2020, an active fixation single coil shock lead was deployed easily to the right ventricle apex and an active fixation atrial lead to the right atrial appendage. The CS was cannulated with the use of the BIOTRONIK hook CS catheter together with an Amplatz left (AL2) with relative ease. A balloon venogram was performed. In the left anterior oblique view (LAO), this showed one suitable target. The suitable target had a corkscrew origin which we were able to navigate but unfortunately, it also had a very narrow partially collateralised section about 1 inch down the vessel which prevented passage of a standard quadripolar lead. We were able to get a PT choice wire down all the way through the collaterals into the main CS again but unfortunately, even this manoeuvre did not provide the support required to pass through the narrow portion, this is illustrated in Figure 1.
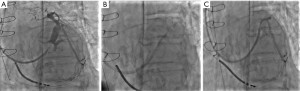
This gentleman was on lifelong anticoagulation and as such his two main options were either a re-attempted placement with the Worley Kit which we thought had a 50:50 chance of success, or a transseptal puncture and endocardial LV lead implant which would have a much higher chance of a good LV lead position. The prospect of hunting for an epicardial vessel only to find out that sits on top of the scar from his aneurism repair was excluded and a trans-septal approach was arranged to implant an endocardial LV lead implant. Timeline of the patient’s history is provided in Figure 2.

Schematic presentation of the procedure
The procedure was performed under local anaesthetic and mild sedation. Both groins and the left pectoral area were prepared. Venous access was gained via extra thoracic subclavian ultrasound (US)-guided right femoral access was gained successfully without any complication.
Trans-septal puncture
Trans-septal puncture was performed with SL1 then exchanged for Agilis via the right femoral (veine). A high transseptal puncture gives a better run with lead and straight stylet. This was performed with a Brockenbrough needle (St. Jude Medical, St. Paul, MN, USA). The guidewire was advanced in the left upper pulmonary vein (LUPV) and Agilis and SL1 were guided to the left atrium (LA) through the same aperture. A 10 mm × 40 mm × 13 mm balloon inflation across the interatrial septum (IAS) was performed next to Agilis to create a large aperture to allow passage to the LV lead.
Trans septal LV lead placement
A pre-pectoral pocket was fashioned, venous access was gained, and active fixation right ventricle (RV) leads were advanced to the right atrium (RA). A 6 French multipurpose angio sheath (MPA) guider with 25 mm GooseNeck snare was passed through Y connector and pressure line to sidearm. Guidewire was advanced beside the MPA catheter in Agilis up the centre of trans-septal sheath under fluoroscopy guidance. The LV lead was advanced sharply J curved to the RA from the superior vena cava (SVC). The tip of the lead was grabbed by the GooseNeck snare, the stylet was pulled back from within the RV pacing lead, leaving it flexible and steered to the IAS. Crossed MPA catheter and pacing lead alongside the guidewire that is across the IAS over in to the LA followed up by the Agilis sheath into the LA once across. Agilis was deflected and 0.035 wire was advanced to the LV followed by the MPA catheter through the mitral valve and guided to the LV. GooseNeck snare was released, and stylet was advanced within the pacing lead which allowed active fixation of the lead endocardially.
Pacing thresholds and stability tests were performed, sheaths were removed, and the lead was secured in the pre-pectoral pocket and attached to a CRT device.
At least 5–6 apical segments were tested but were silent with no electrical signals or recordable threshold but eventually, we managed to get basally with good electrical parameters, illustrated in Figure 3.

A 3-month follow-up echocardiogram was arranged, this showed, mild improvement to the overall ejection fraction, estimated to be around 25%. There was also only mild improvement in overall patient’s overall conditions, however, given his multiple other co-morbidities, his symptoms are considered to be multi-factorial rather than pure cardiac. Post-TSLV lead implantation follow-up checks were all normal with good electrical parameters and appropriate biventricular pacing.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Trans-septal endocardial LV lead placement is an alternative technique used in cases where a trans-venous approach via the CS is challenging or impossible, but due to lack of clinical trials and evidence, this technique is underused. The risk of system embolization remains a cause for major concern, in a study by Gellér et al., it was demonstrated that, even though an effective approach, it was associated with a higher rate of thromboembolic cerebrovascular accident (CVA) of 7% compared to general background rate (2). However, other studies suggest that with appropriate anticoagulation, this risk was significantly lower (16). This makes the case for ongoing trials targeting the safety and efficacy of TSLV lead.
So far, small and mainly retrospective studies have been carried out to analyze the safety and long-term effects of TSLV. In a comparative analysis by Moriña-Vázquez et al., the TSLV approach was safe and a better alternative than the surgical epicardial LV lead (17). Scott et al., demonstrated that ECG markers of repolarization post-trans-septal endocardial LV lead were associated with less arrhythmogenic repolarisation characteristics (18). Patel et al., presented a case series of five procedures using the snare coupling technique as a simple and reliable approach to implant an endocardial LV lead (19).
On the other hand, in recent years, with the emergence of conduction system pacing and mainly the rapid development of left bundle area pacing as a safe alternative to CRT, we have taken a step away from TSLV endocardial lead implant and instead choose conduction system pacing in patients with failed CS approach. However, in select patients, for instance, this particular case, who had a large LV aneuresymectomy, a left bundle area pacing would not guarantee success and HIS pacing was not considered as an alternative option by the consultant physicians. In the future, randomized controlled trials will be necessary to provide more evidence regarding safety and reliability of conduction system pacing however, so far, studies have been very promising.
Conclusions
In select patients, a TSLV endocardial lead implantation is a safe alternative following a failed traditional LV lead implant via the CS. In our patient, although, following, an endocardial LV lead implant, there was not significant symptomatic relieve and remained in functional New York Heart Association (NYHA) class III. One would assume that in a patient with extensive ischaemic background, the fact that his electrical dyssynchrony is now resolved, and there was mild improvement in echocardiographic parameters, demonstrates the effectiveness of the procedure.
Patient’s perspective: at his follow-up, the patient was happy that he was not admitted to the hospital with decompensated heart failure again since the TSLV lead implant.
Acknowledgments
We thank the work of the cardiac chief physiologists from Derriford Hospital, Plymouth NHS Trust for their part in device optimisation and programming.
Funding: The study was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-23-109/rc
Peer Review File: Available at https://acr.amegroups.com/article/view/10.21037/acr-23-109/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-23-109/coif). K.G. reports the funding from Health Education England East Midlands and United Lincolnshire Hospitals NHS Trusts. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- León AR, Abraham WT, Curtis AB, et al. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol 2005;46:2348-56. [Crossref] [PubMed]
- Gellér L, Salló Z, Molnár L, et al. Long-term single-centre large volume experience with transseptal endocardial left ventricular lead implantation. Europace 2019;21:1237-45. [Crossref] [PubMed]
- van Gelder BM, Scheffer MG, Meijer A, et al. Transseptal endocardial left ventricular pacing: an alternative technique for coronary sinus lead placement in cardiac resynchronization therapy. Heart Rhythm 2007;4:454-60. [Crossref] [PubMed]
- Morgan JM, Biffi M, Gellér L, et al. ALternate Site Cardiac ResYNChronization (ALSYNC): a prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J 2016;37:2118-27. [Crossref] [PubMed]
- Navia JL, Atik FA, Grimm RA, et al. Minimally invasive left ventricular epicardial lead placement: surgical techniques for heart failure resynchronization therapy. Ann Thorac Surg 2005;79:1536-44; discussion 1536-44. [Crossref] [PubMed]
- Nelson KE, Bates MG, Turley AJ, et al. Video-assisted thoracoscopic left ventricular pacing in patients with and without previous sternotomy. Ann Thorac Surg 2013;95:907-13. [Crossref] [PubMed]
- Miller AL, Kramer DB, Lewis EF, et al. Event-free survival following CRT with surgically implanted LV leads versus standard transvenous approach. Pacing Clin Electrophysiol 2011;34:490-500. [Crossref] [PubMed]
- Vijayaraman P, Herweg B, Ellenbogen KA, et al. His-Optimized Cardiac Resynchronization Therapy to Maximize Electrical Resynchronization: A Feasibility Study. Circ Arrhythm Electrophysiol 2019;12:e006934. [Crossref] [PubMed]
- Vijayaraman P, Ponnusamy S, Cano Ó, et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results From the International LBBAP Collaborative Study Group. JACC Clin Electrophysiol 2021;7:135-47. [Crossref] [PubMed]
- Wu S, Su L, Vijayaraman P, et al. Left Bundle Branch Pacing for Cardiac Resynchronization Therapy: Nonrandomized On-Treatment Comparison With His Bundle Pacing and Biventricular Pacing. Can J Cardiol 2021;37:319-28. [Crossref] [PubMed]
- De Maria E, Ziacchi M, Diemberger I, et al. Leadless left ventricular endocardial pacing: a real alternative or a luxury for a few? Cardiovasc Diagn Ther 2018;8:530-3. [Crossref] [PubMed]
- Gamble JHP, Herring N, Ginks M, et al. Procedural Success of Left Ventricular Lead Placement for Cardiac Resynchronization Therapy: A Meta-Analysis. JACC Clin Electrophysiol 2016;2:69-77. [Crossref] [PubMed]
- Ypenburg C, Schalij MJ, Bleeker GB, et al. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J 2007;28:33-41. [Crossref] [PubMed]
- Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329-38. [Crossref] [PubMed]
- Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53. [Crossref] [PubMed]
- Rademakers LM, van Gelder BM, Scheffer MG, et al. Mid-term follow up of thromboembolic complications in left ventricular endocardial cardiac resynchronization therapy. Heart Rhythm 2014;11:609-13. [Crossref] [PubMed]
- Moriña-Vázquez P, Roa-Garrido J, Fernández-Gómez JM, et al. Direct left ventricular endocardial pacing: an alternative when traditional resynchronization via coronary sinus is not feasible or effective. Pacing Clin Electrophysiol 2013;36:699-706. [Crossref] [PubMed]
- Scott PA, Yue AM, Watts E, et al. Transseptal left ventricular endocardial pacing reduces dispersion of ventricular repolarization. Pacing Clin Electrophysiol 2011;34:1258-66. [Crossref] [PubMed]
- Patel MB, Worley SJ. Snare coupling of the pre-pectoral pacing lead delivery catheter to the femoral transseptal apparatus for endocardial cardiac resynchronization therapy: mid-term results. J Interv Card Electrophysiol 2013;36:209-16. [Crossref] [PubMed]
Cite this article as: Farhangee A, Davies E, Haywood G, Gaughan K, Mindrila I. Trans-septal left ventricular endocardial lead in a patient with extensive anterior myocardial infarction and left ventricle (LV) apical endoventriculoplasty using a Vascutek patch—case report. AME Case Rep 2024;8:106.


